Editor
Investors in Novacea Inc. prior to its reverse merger with Transcept Pharmaceuticals Inc. three years ago might be forgiven for saying, "I told you so."
At the time of the deal, Novacea was sitting on about $90 million left over after the Phase III failure of prostate cancer drug Asentar. It was rumored that nearly 100 privately held biotechs vied for a chance to reverse merge with the cash shell, and Transcept won out – much to the dismay of Novacea's investors.
On the conference call announcing the deal, Novacea investors complained that the firm was giving away its cash to an entity that still had to face an increasingly cautious FDA with a product that lacked external validation. They raised concerns about cross-ownership issues, called the deal "very, very bad for the company's public shareholders," and pushed the stock down 24.3 percent. (See BioWorld Today, Sept. 3, 2008.)
Now that the FDA has issued a second complete response letter for Trancept's insomnia drug Intermezzo (zolpidem tartrate), it looks like investors were right to be concerned. (See BioWorld Today, July 14, 2011.)
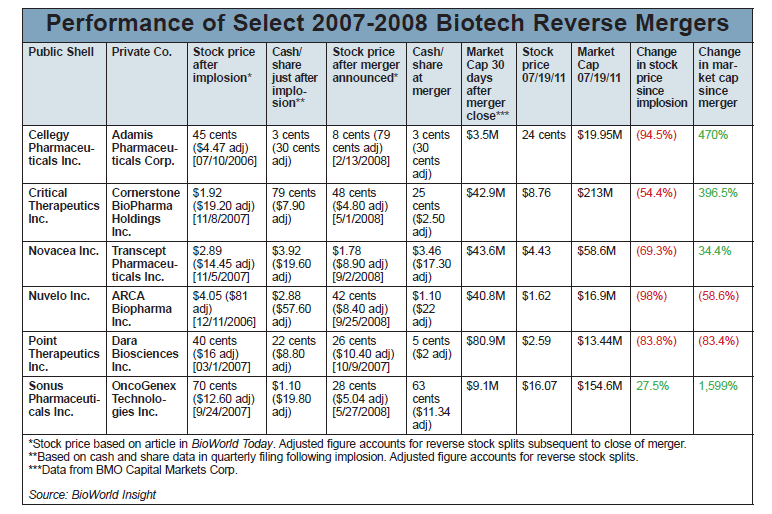
According to a BioWorld Insight analysis, the stock is now down 69 percent from where it was just after Novacea's Asentar imploded. And Novacea was trading below its cash value at the time; the decline from what investors would have received had Novacea liquidated back then versus what their Transcept shares are worth today is 77 percent. (See the chart on page 7.)
But the deal wasn't bad for everyone involved. In fact, comparing the market capitalization of the merged entity just after the deal closed to the current market cap shows a 34 percent increase in value, even after the Intermezzo complete response letter. Running the numbers before that recent setback would have shown a whopping 238 percent value creation.
Success Versus Failure
There are some reverse mergers that look good in every light. Cougar Biotechnology Inc. is the biotech example most-often cited: The firm reversed merged with shell SRKP 4 Inc. back in 2006, saw its stock rocket 150 percent in three years thanks to good data for prostate cancer drug abiraterone acetate, and was sold to Johnson & Johnson for nearly $1 billion. (See BioWorld Today, April 10, 2006, and May 26, 2009.)
More recently, there was OncoGenex Pharmaceuticals Inc., which went public in 2008 via a reverse merger with Sonus Pharmaceuticals Inc. following the Phase III failure of Sonus' Tocosol Paclitaxel. Thanks to great data with OncoGenex's antisense pipeline, including lead drug custirsen for prostate cancer, the merged firm has seen nearly a 1,600 percent increase in market cap, and even original Sonus investors have seen a positive return. (See BioWorld Today, May 29, 2008, and Dec. 22, 2009.)
But such glowing examples are scarce. On the whole, biotech reverse mergers tend to be a bad deal for investors in the public shell. That's why, in recent years, investors have fought such transactions. Investor pressure put the kibosh on a proposed 2009 merger between Australian biotechs Progen Pharmaceuticals Ltd. and Avexa Ltd., while Deerfield Management prevented shell firm NitroMed Inc. merging with Archemix Inc. Shareholders of VaxGen Inc. voted down two reverse merger proposals – one with Raven Biotechnologies Inc. and another with Oxigene Inc. – before finally agreeing to merge with diaDexus Inc. (See BioWorld Insight, Feb. 28, 2011.)
Whether or not reverse mergers are good for investors in the new merged entity is hit or miss: market caps tend to go either way up or way down.
Steven Bronson, founder, chairman and CEO of investment banking firm Catalyst Financial LLC, said the success of a reverse merger is driven by two factors. The first, and most important, is "execution of the business model," he said. The shell company is making a bet that the technology being developed by the private firm will work, and in the biotech industry, that's always a risky bet.
So you have outcomes like ARCA Biopharma Inc., which reverse-merged with Nuvelo Inc. after the failure of Nuvelo's clot-busting drug alfimeprase. Shares of Nuvelo gained when the merger was announced, perhaps because ARCA's beta-blocker Gencaro (bucindolol) was already under FDA review for heart failure. But the FDA slapped Gencaro with a complete response letter requesting another trial, and shares plummeted. (See BioWorld Today, Sept. 26, 2008, and March 12, 2010.)
The second factor Bronson cited as critical to the success of a reverse merger is sponsorship. Unlike initial public offerings (IPO), reverse mergers don't involve investment bankers trotting management around to meet with institutional investors, raising awareness and creating a shareholder base. Thus, like firms that go public on a foreign exchange and then transfer to the OTCBB, or those that go public via the Form 10 pathway, visibility and volume can be very low at first.
But "ultimately, it's about the success of the business," Bronson said.
Annette Grimaldi, managing director with BMO Capital Markets Corp., agreed. The most important thing management teams of the shell company can do to ensure the success of the reverse merger is extensive due diligence, she said, adding "the key is choosing to combine with a company that has a good chance."
Drawbacks Versus Benefits
By that logic, reverse mergers, despite their bad reputation, might not necessarily be more doomed to failure than any other biotech investment. Yet Grimaldi noted that, historically, some private companies have opted for reverse mergers because they were not strong enough for a traditional IPO, resulting in poor aftermarket performance as a result of negative selection bias rather than a problem inherent in the reverse merger structure.
Reverse mergers do come laden with their own, unique baggage. Many failed biotech shells have disgruntled shareholders who may not be interested in the private company, and it takes time for them to cycle out of the stock. Shells also tend to have a largely retail shareholder base, and it takes not only time, but adequate price appreciation and volume to attract institutions. There's also the lack of sponsorship and requirement of shareholder approval, as previously noted, and the fact that the shell might have debts or other liabilities.
Reverse mergers will also soon face new hurdles from Nasdaq. The exchange has filed three proposals that would result in more stringent listing requirements for firms that go public through the reverse merger process.
First, reverse merger firms will not be eligible to apply for Nasdaq listing until six months after the merged entity submits audited financial statements to the SEC. Second, the merged company's stock must have a closing bid price of $4 or more for at least 30 of the 60 trading days immediately preceding the application for Nasdaq listing. Third, at least two financial reports related to the combined entity must be filed with the SEC prior to listing on Nasdaq.
The proposals are under review at the SEC. Although they arose from recent problems with sketchy Chinese companies using reverse mergers to gain a U.S. listing, Nasdaq spokespersons said they would apply to all reverse mergers in the future. That includes private biotechs that merge into a Form-10 shell or an OTC-listed shell with the intention of transferring to Nasdaq, although the changes would not impact biotechs that merge into a Nasdaq-listed shell.
Despite the many challenges, there are benefits to reverse mergers as well. In a recent research note, Grimaldi wrote that reverse mergers are less susceptible to market conditions than IPOs, and that the separation of the listing process from a financing gives the company more financing flexibility. Indeed, in the biotech world, many shells bring a hefty cash balance to the table, removing the need for subsequent financing in the short term.
Then Versus Now
For these are other reasons, reverse mergers continue to be a popular strategy in biotech, with plenty of firms hoping to be the next Cougar or OncoGenex.
Last month, private firm Allozyne Inc. announced plans to merge with struggling Nasdaq-listed biotech Poniard Pharmaceuticals Inc., while Synageva BioPharma Corp. moved to reverse merge with Nasdaq-listed Trimeris Inc. (See BioWorld Today, June 14, 2011, and June 24, 2011.)
Earlier this year, Radius Health Inc. said it had begun the process of going public through a reverse merger with unregistered MPM Acquisition Corp. VistaGen Therapeutics Inc. inked a deal to merge with OTCBB-listed Excaliber Enterprises Ltd., and IntelliCell Biosciences Inc. announced plans to merge with OTCBB-listed Media Exchange Group. Can-Fite BioPharma Ltd. also agreed to spin off its ophthalmic indications in a reverse merger deal with OTCBB-listed Denali Concrete Management Inc. (See BioWorld Today, May 25, 2011.)
Late last year, Pathfinder LLC decided to go public via a reverse merger with OTCBB-listed SyntheMed Inc., Genesis Biopharma Inc. went public through a reverse merger with OTCBB-listed Freight Management Co. and Insys Therapeutics Inc. reverse merged with Pink Sheets-listed NeoPharm Inc.
As long as the IPO window remains largely closed, biotechs will likely continue to pursue alternative pathways to public listing, such as reverse mergers and Form-10 filings. (See a related story in this issue.) And as long as biotechs continue to have a high failure rate, there should be no shortage of publicly listed biotech shells for private firms to merge into.
Perhaps even Transcept, which itself went public via a reverse merger with a shell, could one day wind up serving as a shell for the next private biotech seeking a public listing. As of now, Transcept has said it is continuing to pursue a path forward for Intermezzo and develop TO-2061, a low-dose formulation of ondansetron that recently began Phase II trials as an adjunctive for obsessive compulsive disorder.
But Transcept does have a Nasdaq listing, not to mention $59.6 million of cash, equivalents and marketable securities as of June 30. That package probably looks pretty enticing to many private biotechs finding themselves running on venture fumes and shut out of the IPO window.