BB&T Contributing Editor
BOSTON The 28th annual Global Growth Conference, sponsored by Canaccord Adams (Vancouver, British Columbia), was held here in early August and featured about 350 growth companies from a wide swath of the economy. Although medical technology was dwarfed by many other economic areas, it still was a very prominent participant and drew strong interest from money mangers eager to hear timely updates and "pitches" from these companies.
On the eve of the conference, highly-regarded Canaccord med-tech analyst Jason Mills published an impressive and comprehensive report titled A-Fib: Near a Tipping Point, in which he asserted that the med-tech companies serving this sector are "just scratching the surface of a $13 billion market opportunity."
Mills estimated that within the atrial fibrillation market, the catheter ablation sector, basically targeting intermittent or paroxysmal Afib (PAF) in developed countries (U.S., Europe, Japan) is only 3.6% penetrated. He also believes that surgical ablation, targeting mostly persistent or chronic Afib (CAF), is less than 2% penetrated.
CEO Dave Drachman of Atricure (West Chester, Ohio) said that "we are a company on the move" in the Afib space, with the leading market share in the surgical ablation arena. He claimed a 52% share, nearly double his next largest competitor, Medtronic (Minneapolis), and quadruple the share of ATS Medical (ATS; also Minneapolis).
Drachman defined the surgical ablation market in two buckets "concomitant" (i.e., in tandem with another open-heart procedure such as a CABG or valve replacement) and "stand-alone" (a less-invasive procedure solely for treating Afib) — and indicated that his company has been involved in nearly 55,000 procedures since entering this market in early 2003.
He said that the latter opportunity was vastly larger than concomitant, estimating it at about $2 billion. Stand-alone is particularly attractive because it is buoyed by a lucrative reimbursement level that makes it "the most profitable cardiovascular procedure in the hospital today."
Based on company data and information provided on its quarterly conference calls with the financial community, Atricure's revenue per procedure in this category typically reaches or exceeds $9,000, while a concomitant operation yields about $3,000.
Atricure was a pioneer in the Afib surgical space and Drachman noted that the company continues to be strongly committed to innovation and new products. Its aggressive R & D spending, which has accounted for about 18% of first-half 2008 worldwide revenue, is highly productive, based on its pipeline of new products.
These additions, which will roll out over the next 12 to 18 months, include disposable cryo-energy probes, an expanded platform for its already very successful Coolrail linear ablation pen, a next generation isolator clamp and a left atrial appendage (LAA) clip.
Drachman defined the latter as a potential "franchise product" based on a huge untapped market and its apparent superiority over the currently available and aging stapling or suturing approaches.
Although a controversial issue, many experts believe that occluding the LAA will reduce the risk of an embolic stroke following an Afib ablation. AtriCure recently received FDA approval to begin enrollment in its EXCLUDE left atrial appendage occlusion trial, which will need to recruit 60 patients, 30 of whom will be followed for six months.
A 510(k) filing could occur in mid-2009, implying a possible FDA clearance and domestic launch in late 2H09. In the Canaccord report, Mills said, "We expect the clip to become a 'staple' within AtriCure's minimally invasive product suite."
Drachman pointed out that his company has been making enormous financial strides with its "disciplined spending," noting that 1H08 revenue surged 23% over the prior year, with a modest 4% increase in expenses. The company showed a loss of about $5.2 million in the first half of 2008 on revenue of $28.4 million. It hopes to achieve break-even status and positive cash flow sometime in 2009.
"We are all about execution and are totally committed to profitability," he said.
The ebullient sentiments of Mills and Drachman on the Afib opportunity were heartily supported by the CEO of ATS, Mike Dale, who said that it is "the biggest opportunity in cardiac surgery today." He indicated that this "truly emerging market" is only about $151 million today, but represents a $1 million to $3 billion overall opportunity.
Whereas Atricure was described by Drachman as a "pure play," with 100% of its sales generated by Afib, ATS is broadly diversified within cardiovascular surgery. In launching coverage on ATS earlier this year, Mills had estimated that about one-quarter of its global revenue of roughly $65 million in 2008 would be generated from its A-Fib efforts.
Atricure derives a significant percentage of its revenue from the minimally-invasive, beating heart stand-alone Afib procedure and uses radio frequency energy. Conversely, the vast majority of ATS's Afib revenue today is coming from its stopped-heart concomitant procedures using cryo-energy.
ATS is a newcomer to the stand-alone market and derives a modest amount of revenue from its stopped-heart, robotic or endoscopic, right-sided incisions. These early cases are being performed by either robotically or endoscopically competent cardiovascular surgeons or surgeons skilled in mitral valve repair.
Dale alluded several times to the company's strong relationship with and support from its new medical director and noted cardiac surgeon James Cox, MD, of the Washington University School of Medicine (St. Louis). Cox, who pioneered open-chest atrial fibrillation surgery decades ago, has stated on many occasions that "the only sure way" to achieve normal rhythm in AF patients is to cryo-ablate the coronary sinus.
Dale was clear in the breakout session that ATS "has beating-heart aspirations," but candidly admitted that neither Cox nor his company had a "definitive solution" as yet. Some cardiovascular surgeons believe that extremely cold cryo energy is incompatible with the circulating warm blood that exists in a beating-heart procedure. Thus, a solution will be technically challenging and require considerable development efforts.
In the past several years, under the leadership of Dale, ATS has been transformed. Just one year ago, it derived more than 80% of its global revenue from the essentially mature mechanical heart valve market. In 2Q08, that contribution had dropped to less to 64%.
This improved balancing of its revenue mix was mainly due to its June 2007 acquisition of the surgical cryo business of CryoCath (Montreal) and the September 2006 purchase of privately-owned, venture capital-backed tissue valve maker 3f Therapeutics (Lake Forest, California).
ATS is currently registering robust growth in all three new areas. The tissue valve product line is been buoyed by the launch of the 3f stent-less aortic tissue valve in Europe. Valve repair is benefiting from the introduction of two new mitral valve annuloplasty rings, while Afib revenues are enjoying nice gains from a better focus and broader distribution.
On the company's recent conference call with analysts, Dale indicated that FDA approval for its 3f tissue valve could come shortly. This would be a big boost to the company, enabling it to compete in the far larger domestic tissue valve market. He estimated the global tissue valve market at $771 million in 2008.
As a result of ATS's aggressive acquisition strategy, its "superior product solutions" and its single-minded focus on cardiac surgery, Dale said he believes that his company is on the verge of "dramatic and significant leverage" in its operations.
Companies participating in the peripheral and cardiovascular space are enjoying very solid growth. A prime example is Spectranetics (Colorado Springs, Colorado), which recently reported that its 2Q08 global revenue surged 31% to nearly $27 million. This rate of gain matched the increase it experienced in fiscal 2007 compared to fiscal 2006.
CEO/President John Schulte told participants at the Canaccord Adams conference that "we are unique in the cardiovascular space — we are the only company with an FDA approval for both peripheral vascular intervention (PVI) and lead removal."
In the most recent quarter, its PVI business grew 26% and accounted for roughly two-thirds of the company's disposables revenue, while lead management, which accounted for one-third of disposables revenue, soared 50%.
Further evidence of the health of Spectranetics' business is that its installed base of 308 nanometer excimer lasers continues to steadily grow, having doubled in the past four years to reach 800. In the second quarter alone, the company reported 33 net laser systems placements, along with eight outright sales.
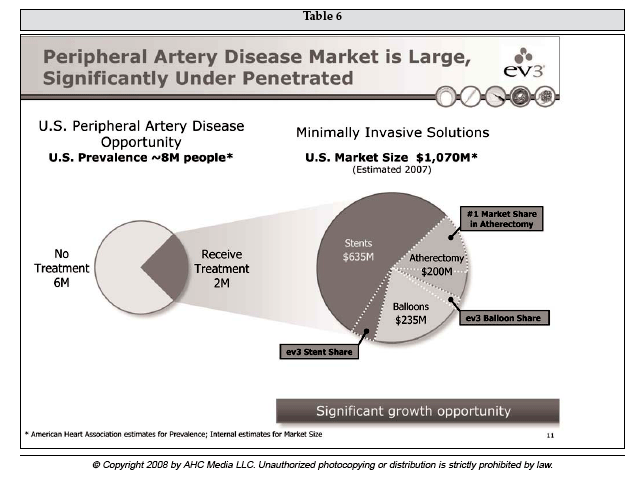
Schulte, who has been CEO for about five years, pointed out that both its key markets are substantially underpenetrated. For example, as shown in Table 5 he said that in the PVI sector, while an estimated 12 million people are afflicted with peripheral artery disease (PAD), only 580,000 or about 5% are treated annually. Of the patients treated, 430,000 receive endovascular procedures, with only 80,000 or 19% managed with atherectomy devices such as Spectranetics' laser.

He indicated that various atherectomy devices, which include the Spectranetics laser and associated catheters, could easily grow to half of the endovascular PAD therapy market, garnering share from both balloon angioplasty and stents.
In a similar vein, the lead implant business appears to have major upside potential. To wit, Schulte said that of the estimated 102,000 annual active defibrillator or pacemakers leads that are removed from service due to infection, malfunction, occlusion or upgrading, only about 15,000 per year are actually extracted.
"I believe that this market is hugely underserved and that it offers our company a tremendous opportunity," he said Backing up these words with action, in early 2008 Spectranetics developed a dedicated lead removal sales organization, with a 31-person sales staff now in place. By year-end, this team will grow to about 40 reps. It is focusing its marketing message on changing behavior from capping of old leads to lead removal.
Clinical support for laser lead removal is growing. An article, titled "Large, Single- catheter, Single-operator Experience with Transvenous Lead Extraction: Outcomes and Changing Indications," in the April issue of HeartRhythm, a highly-regarded publication directed mainly to electrophysiologists, concluded that laser-assisted extraction of leads is safe and effective. The study was conducted at Brigham and Women's Hospital (Boston) and represented a seven-year retrospective analysis of data from 498 lead extraction procedures involving 975 leads.
Compared to the PAD marketplace, which has become much more competitive in the past couple of years, lead removal competition is relatively benign and if Spectranetics is successful in growing this market to its avowed goal of 50,000 leads removed per year, it will be a major beneficiary.
Schulte also outlined a very aggressive clinical program that has been designed to broaden the company's available PVI market. He outlined three ongoing trials — PATENT, SALVAGE and TAAMI — which he believes will fuel future growth.
The first two clinical trials are designed for the treatment of superficial femoral artery (SFA) in-stent restenosis, an opportunity that Schulte called "compelling." He said that "no good treatment options exist in this market segment, which is estimated to be at least 25% of all SFA procedures." PATENT is a prospective 100-patient study being conducted at up to 10 sites in Germany.
SALVAGE, which is being conducted by both Spectranetics and privately-owned W.L. Gore Associates (Flagstaff, Arizona) in the U.S., also is a 100-patient prospective, multi-center trial. Whereas PATENT will solely rely on Spectranetics' Turbo-Booster and Turbo catheters, SALVAGE will evaluate a combination of the laser and the Gore Viabahn endoprosthesis with a heparin bioactive surface. This device is currently the only stent graft approved for treating PAD in the SFA.
TAAMI is a 200-patient randomized trial involving major medical centers in Poland that will enroll two treatment groups and in the most complex myocardial infarction patients — those with a large thrombus burden presenting with ST wave elevation myocardial infarction.
With rapidly growing revenue growth, which has been proceeding at a 34% compounded annual rate the past three years, Schulte said, "We expect our profitability to improve" and pointed out that at a $30 million quarterly run rate, his company will be operating in the black.
Another company operating in the PVI space is ev3 (Plymouth, Minnesota), whose new relatively CEO Bob Palmisano could not attend but was ably replaced at the Cannacord Adams podium by CFO Pat Spangler and Director of Communications Julie Tracy.
ev3 derives roughly 70% of its current revenue from PVI, with the balance generated by its interventional neuroradiology activities. Within PVI, ev3 believes that with the acquisition of FoxHollow Technologies (Redwood City, California) in October 2007, it is the leading player in the U.S. atherectomy market, which Tracy said is a $1.1 billion annual market. Despite fierce competition, ev3's goal is "to build the market rather than grow through taking market share from competition." Table 6 displays ev3's current view of the PAD market opportunity.
To achieve this goal, it is aggressively expanding its atherectomy offerings with the SilverHawk with MEC TEC (micro-efficient compression technology). Utilizing precision laser-drilled micro vent holes, it can capture up to 30% more tissue compared to the older Silverhawk device.
A second offering in atherectomy is the RockHawk device for removing calcified SFA lesions. It already has been approved for surgical use in the U.S. and ev3 hopes to launch an IDE trial, dubbed DEFINITIVE, in 4Q08. The trial will enroll 100 patients and will include the use of the SpiderFX embolic protection device, with the goal to broadening the indication to endovascular use as well.
Similar to Spectranetics, ev3 is hoping to drive its PVI growth with broad clinical initiatives. Spangler noted several clinical trials that will be important to the future. Enrollment is continuing in both the U.S. and Europe in its DURABILITY stent trials.
In addition to the RockHawk endovascular trial, ev3 will soon begin enrollment in a domestic, post-market study for SilverHawk, which never underwent a rigorous clinical examination under the auspices of FoxHollow. Under Palmisano's veteran leadership, ev3 has begun to rebuild investor confidence, with numerous achievements since he was appointed CEO in April. In 2Q08, global sales exceeded its guidance, gross margins expanded, expense ratios have improved and its EBITDA loss has been reduced.
Both Spangler and Tracy praised Palmisano, noting that he has provided "focus and discipline" to the organization.
Another presenting publicly-traded company was Enteromedics (St. Paul, Minnesota), whose CEO, Mark Knudson, said that his company's technology addresses a huge market and will be successful because it "targets the shortcomings of existing obesity treatments."
According to Knudson, the limitations of today's two key obesity therapies — gastric bypass and laparoscopic banding — are so significant that only 1.5% of the approximately 17 million obese Americans seek surgery. He specifically estimated the number of bariatric surgeries for the year 2007 at 205,000.
Knudson noted that there are several unmet needs in this market. He indicated that its innovative solution, the Maestro intermittent neuroblocking system, would address over 80% of the potential surgical candidate market.
This procedure involves the laparoscopic implantation of a pacemaker-like device, whose leads are lodged into the vagus nerve. Called VBLOC therapy, the multiple layers of therapeutic intermittency of high-frequency, low-energy electrical impulses, "block" the vagus nerve during the waking hours. Most patients report that they "push away from the table sooner," because they feel satiated.

