CDU Contributing Editor
SAN DIEGO, California – In the past several years, there has been a rapidly growing realization that congestive heart failure (CHF) represents a huge health care problem and a massive market opportunity for both the pharmaceutical and the medical device industry. Indeed, at the annual scientific sessions of the North American Society of Pacing and Electrophysiology (NASPE; Natick, Massachusetts) held here last month, several speakers cited the enormity of the CHF health care problem. For example, CHF, afflicting about 550,000 new patients per year and 1% of the over-65 age group, now represents the single largest expense for the Medicare program (5% of total costs) and perhaps most importantly, has a staggering 50% five-year mortality rate. It results in more than 1 million hospital discharges per year, with 79% of this total represented by re-admissions. As a result, one witty physician at the NASPE gathering termed CHF patients "the ultimate frequent flyers."
There is unanimity in the cardiology community on the magnitude of the CHF problem, as well as a realization that implantable devices will play a pivotal role in the management of these severely ill patients. For example, Dr. William Abraham, professor of medicine and chief of the division of cardiovascular medicine at the University of Kentucky College of Medicine (Lexington, Kentucky) and the principal investigator for one of the key CHF device clinical trials (MIRACLE), remarked that, following several decades of lifestyle and medical management as the prime weapons against CHF for the decade of 2000-2010, "we have now entered the device era in the management of CHF." While most electrophysiology (EP) physicians would not disagree with that contention, there is considerable controversy and debate on the most appropriate role for devices to treat CHF.
Specifically, the debate centers around whether one of the most exciting new technologies, biventricular pacing (BVP), also referred to as cardiac resynchronization therapy (CRT) is sufficient for most patients or whether a device with back-up implantable cardioverter-defibrillator (ICD) technology should be implanted instead. BVP, which uses pacemaker technology to deliver synchronized electrical stimulation to three chambers of the heart, can restore the rhythmic contractions of the heart. The major difference between these products is that the latter offers an on-board ICD, which can terminate a potentially lethal ventricular tachycardia that, if unchecked, can cause sudden cardiac death. In several clinical trials, CRT has been shown to be both very safe and effective in alleviating symptoms and improving the quality of life of CHF patients, particularly those most severely-afflicted (i.e., New York Heart Association Class III and Class IV).
Two BVP devices are now FDA-approved, the InSync from Medtronic (Minneapolis, Minnesota) and the Contak-CD from Guidant (Indianapolis, Indiana). Medtronic's device does not yet offer that ICD back-up capability for the U.S. market, but based upon a successful March FDA Circulatory System Devices Panel meeting, it should have a comparable product available for U.S. marketing soon.
So, what is the appropriate role of these devices to manage CHT and where is it clinically opportune to use and BVP device with ICD back-up? In a lively debate titled "All Patients Who Receive Cardiac Resynchronization Therapy Should Also Receive an ICD," a noted heart failure specialist, Dr. Lynne Warner Stevenson, director of the cardiomyopathy and heart failure program at Brigham and Women's Hospital (Boston, Massachusetts), squared off against renowned electrophysiologist Dr. Leslie Saxon, who is director of implantable device services at the University of California San Francisco Hospital (San Francisco, California).
Stevenson contended that while there have not been any clinical trials to support the use of a combination BVP/ICD device, nevertheless she felt that because high-risk patients desire to live longer and need protection against a SCD episode, these patients should receive a combination device. Interestingly, in a talk the next day, she indicated that it would be "very hard to prove survival benefits for CRT" because it would require a huge number of patients and patient enrollment in a randomized trial (where half the patients would not get a CRT implant) would be very difficult.
On the other hand, Saxon, one of the key physicians in the field of implantable ICDs and CHF devices, noted that the clinical studies to date "have not demonstrated mortality benefits in patients who receive an ICD." Saxon cited an article titled "Primary Prevention of Sudden Cardiac Death in Idiopathic Dilated Cardiomyopathy: The Cardiomyopathy Trial (CAT)," which appeared in the March 18 issue of Circulation. This trial demonstrated that prophylactic ICD implantation in patients with dilated cardiomyopathy of recent onset and impaired left ventricular ejection fraction did not boost their survival time. Saxon concluded her remarks by saying that "we really need to carefully weigh the risks and benefits of CRT and ICD combination therapy compared with CRT alone."
Although neither Saxon nor Warner Stevenson specifically discussed the cost of these devices, several other presenters noted that the enormous cost disparity between these two types of devices needed to be weighed in patient management. For example, Guidant is pricing its Contak-CD at nearly $45,000, a $10,000 premium over its Prizm ICD, while Medtronic's InSync (without ICD backup) sells for about $12,000. Dr. Michael Gold, director of the division of cardiology at the Medical University of South Carolina (Charleston, South Carolina), in a talk titled "Patient Selection and Therapeutic Endpoints," noted that if 1 million CHF devices are implanted over the next five years and that if the cost differential between a BVP vs. a BVP/ICD device is $25,000, then the total cost difference to the U.S. health care system for a million implants would be $25 billion. Although not mentioned by Gold, several EP physicians noted to us that the current Medicare national reimbursement codes are not adequate to cover the cost difference between a combination BVP/ICD and ICD alone, which will become a financial burden on the hospital system.
In his concluding remarks, Gold challenged the electrophysiology community, saying that the current patient selection criteria for CHF devices was "crude" and that it was very important to determine where ICD back-up devices would be required.
The theme that the electrophysiology and heart failure communities have much work ahead was echoed by Dr. Jean Claude Daubert, a highly regarded EP physician from the University of Rennes (Rennes, France). In his talk, titled "Devices for Heart Failure: Where Are We and Where Are We Going," Daubert answered that rhetorical question: "We are at the very early stages of heart failure therapy and we can expect many improvements to come in the future."
The tremendous financial potential of CHF clearly has not gone unnoticed by the Wall Street medical device community. A May report written by Lynn Pieper of the merchant banking firm Thomas Weisel Partners (San Francisco, California) noted that "the CHF market represents a compelling opportunity for device therapy." According to Pieper, there are several factors that will ultimately determine the size of the CRT market for congestive heart failure:
- Current indications for both low power (i.e., without ICD) and high power (with ICD) devices.
- Stratification of the patient population to determine which patients will benefit from what device.
- Reimbursement, especially a payment that is adequate to cover the physician's time.
- Objective data on important issues such as mortality and hospitalization rates.
- The potential to shorten procedure times.
The technical complexity and lengthy procedure times (often three to four hours long) of CRT lead implantation has emerged as an impediment to the rapid adoption of CRT. Indeed, several EP physicians interviewed at NASPE indicated that this was a major issue in the growth of the CRT market. Thus, the recent FDA approval of the Medtronic Attain OTW (over-the-wire) left-heart lead for delivery of cardiac resynchronization therapy is a positive development for this market. The Attain OTW lead is designed to provide easier left-heart access and will be used with both the InSync and InSync ICD systems. According to Dr. Bruce Wilkoff, director of cardiac pacing and tachyarrhythmia devices at the Cleveland Clinic Foundation (Cleveland, Ohio), "The Attain OTW lead lets me choose among more cardiac veins and access them more easily. " With its small lead body size (4 Fr) and distal curves designed for increased steerability and stability, the Attain OTW lead is intended to allow physicians to maneuver it into tortuous anatomies and through small- and medium-sized cardiac veins.
Cardio-Optics (Boulder, Colorado), an early-stage company addressing the lead implantation problem, exhibited for the first time at a NASPE meeting. The company has invented and patented a technology known as Direct Trans-Blood Vision (TBV), which will enable physicians to see through blood (as if it were a clear liquid) and provide direct vision of the tissues and structures inside the body. This technology, if it performs as heralded, could be used in a variety of interventional procedures (EP catheter ablation, coronary angioplasty, septal defect repair) where better visualization could be very beneficial.
The company's initial market focus will be to improve CRT lead implantation. TBV will the simplify the identification of the ostium (opening) of the coronary sinus, where the lead must be placed prior to advancement. Locating the ostium is a challenge in a normal heart but is particularly daunting in the oft-hypertrophied heart of a CHF patient.
Once the ostium has been located, advancing the lead through the coronary sinus to a branch in close proximity to the left ventricle is another significant challenge that can become very time-consuming. A lengthy procedure exposes the EP physician, the patient and cath lab personnel to unduly large doses of potentially harmful fluoroscopic x-rays.
The Cardio-Optics system features a console (about the size of a VCR) that contains the optics and electronics to project an infrared light through the interconnect cable and disposable TBV fiber assembly and onto the tissue structures of interest. An image guide contained within the disposable fiber assembly brings the image of the tissue structures back to the interconnect cable and to the console, where a special infrared camera converts the image to a video output signal. In reality, this system is similar to a flexible endoscopy system, except that it uses a special infrared light and can see through blood.
The company has proof of concept in animals and hopes to initiate human clinical trials shortly.
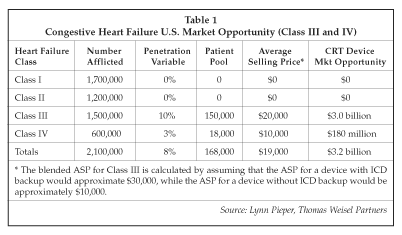
The sizable potential for CRT therapy is displayed in Table 1. In discussing this forecast, Pieper noted that she expects additional positive clinical data will be presented to support expansion of the patient pool and stratify patients appropriately. The data from the current CRT/ICD combination device trials addresses those heart failure patients who also have an indication for an ICD. If favorable data is generated from either the Comparison of Medical Therapy Pacing and Defibrillation in Heart Failure (COMPANION) trial sponsored by Guidant or the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) sponsored by the National Institutes of Health (Bethesda, Maryland), the potential patient population for BVP devices with ICD backup could swell. On the other hand, the two trials may indicate that most patients should be treated with only a BVP device.
 |
The other key market drivers will be new reimbursement codes to adequately cover the device and the physician's implantation time, as well as reduced electrophysiology cath lab procedure times. Cardiovascular Device Update believes that these future market drivers could take months or years to develop, thereby restricting procedure in the early years following market launch.
The model in Table 1 assumes that between 15% and 20% of all CHF patients could qualify as candidates for CHF devices, based on a NYHA class (III-IV), a QRS width of less than 130 milliseconds and an ejection fraction of less than 35%.
Observers of the cardiac resynchronization therapy market believe that Class IV heart failure patients are more apt to receive a biventricular pacer without ICD back-up because this patient group is more susceptible to pump failure than sudden cardiac death. The penetration rate into Class III patients will likely be split between biventricular pacing devices and combination devices and modest penetration into the Class IV patient. Down the road, the large Class II patient population could show a benefit from CRT devices, driven by reduced implant times, increased reimbursement for ICD-based biventricular devices, and favorable data from COMPANION, SCD-HEFT and other clinical trials currently underway.
Another CRT market model, displayed in Table 2, provides actual forecasts for the global CRT market for the 2001-2005 period. As shown, Medtronic and Guidant are expected to dominate, based upon their significant regulatory lead over the third large player in the cardiac rhythm management market, St. Jude Medical (St. Paul, Minnesota). Whereas the other two companies already have domestic CRT product approvals, St. Jude is not expected to enter the U.S. cardiac resynchronization therapy market until late 2003 or 2004.
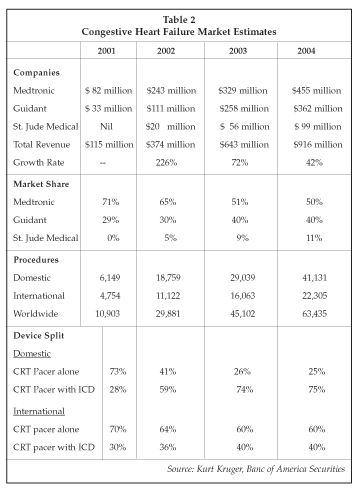 |
The robustness of the CRT market is illustrated by comments contained in Medtronic's recently reported financial results for the fiscal year ended April 30. In that report, CEO Art Collins pointed out that revenue generated from recently introduced heart failure devices are annualizing at about $300 million and have became a major contributor at a rate never before achieved by any new Medtronic product line.

