Neurology/psychiatric
Neurology/psychiatric
Kissei Pharmaceutical presents new orexin OX2 receptor agonists for narcolepsy
Read MoreNeurology/psychiatric
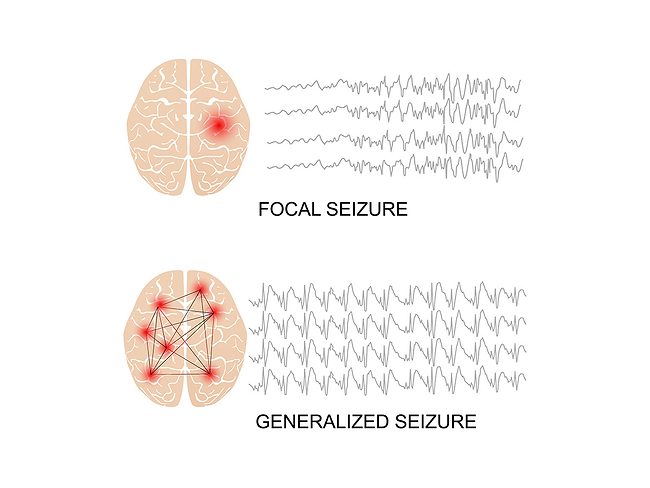
Saniona begins preclinical development of SAN-2355 for focal onset seizures
Read MoreNeurology/psychiatric
French researchers present new Aβ production inhibitors for Alzheimer’s disease
Read MoreNeurology/psychiatric