Medical Device Daily Contributing Writer
ORLANDO — The 58th annual scientific session of the American College of Cardiology (ACC; Bethesda, Maryland) kicked off here early on Saturday morning with a session devoted to "Late Breaking Clinical Trials."
The very first presentation discussed a clinical trial for a novel device for stroke prevention in patients with non-valvular atrial fibrillation (AF). In this study, dubbed the Embolic Protection in Patients with Atrial Fibrillation (PROTECT AF) trial, researchers compared the current standard of therapy, anticoagulation with a blood thinning agent Coumadin (generically warfarin), to a percutaneously implanted device called the Watchman.
It is widely appreciated that patients with AF have a far higher risk of stroke, as the irregular beating of their heart causes blood to pool in the atrium, potentially forming clots which may lodge in the brain. Although anticoagulation with warfarin has been a standard of care for the prevention of clot-related AF strokes for many years, it is used conservatively.
While its best attribute of thinning the blood has been clearly shown to reduce the risk of embolic stroke in patients afflicted with AF, warfarin requires that patients restrict certain physical activities that may cause bleeding or bruising. In addition, the drug interacts with certain foods that hamper its efficacy which, in turn necessitates regular blood tests to be sure it has reached a safe and effective dosage level.
The upshot of these considerable drawbacks is that warfarin compliance is poor and is generally estimated at under 50%. Thus, the results of this trial were keenly anticipated and they did not disappoint. The trial handily met its primary endpoints, as the Watchman reduced the combined risk of cardiovascular death and ischemic and hemorrhagic stroke by more than 30%.
In addition, an impressive 87% of the patients that received the device were able to stop their warfarin therapy.
The manufacturer of this device, privately-owned, venture capital-backed Atritech (Plymouth, Minnesota) was in the news last week, with the announcement that it had concluded a $30 million financing with several VC funds (Medical Device Daily, March 27, 2009).
According to Pete McNerney, a partner with Thomas, McNerney & Partners (Minneapolis) which was the lead investor "We've thought for some time that atrial fibrillation is one of the more significant unmet clinical needs out there.''
Further evidence of Atritech's progress was also disclosed last week, when the company reported that the FDA's Circulatory System Devices panel will review its pre-market application (PMA) on April 23. The company had previously indicated in August that it had submitted its PMA to the FDA.
The Watchman left atrial appendage (LAA) device was developed as a mechanical barrier to seal the orifice of the LAA. which is an outpouching of the left atrium. It is well known in AF patients that if thrombo-embolic material is found in the left atrium, more than 90% is in the LAA.
The Watchman is a three-part system consisting of a trans-septal access sheath, a delivery catheter, and an implantable nitinol device. The system is designed to facilitate device placement via femoral venous access via the transseptal route into the LAA.
In the randomized, prospective and multi-center (international and domestic) PROTECT AF study, 707 patients with non-valvular atrial fibrillation were randomly assigned to closure of the LAA with the Watchman device (463 patients), followed by discontinuation of warfarin, or to long-term treatment with warfarin (244 patients).
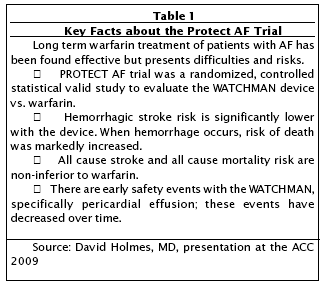
With more than 900 patient-years of follow-up, the study found that the combined rate of stroke (both ischemic and hemorrhagic) and cardiovascular death the primary measures of effectiveness was 3.4 per 100 patient-years in the device group vs. 5.0 per 100 patient-years in the warfarin group. This reduction of 32% was statistically significant.
Speaking at an ACC sponsored press conference, David Holmes, MD, the principal investigator and Scripps professor of medicine at the Mayo Graduate School of Medicine, (Rochester, Minnestoa) said that the efficacy of the Watchman was "dramatically better" than for warfarin..
The safety of the device, which was a primary endpoint, was also met. The trial observed more procedure-related complications in patients treated with the device (8.7 vs. 4.2 per 100 patient-years), with most of the complications related to device implantation. Specifically, the main complications seen were related to pericardial effusion (abnormal accumulation of fluid in the pericardial cavity). This adverse event declined significantly with as the implanting interventionalists gained more experience with the device and also benefited an improvement in the design of the Watchman.
However, after successful implantation of the Watchman and discontinuation of warfarin therapy, complication rates were significantly lower with device therapy.
The researchers concluded that the Watchman is an effective alternative to warfarin therapy for preventing stroke in patients with AF.
"The take-home message is that although there are complications associated with implantation of the device, patients can avoid the need for chronic warfarin therapy, with all its attendant risks," Holmes said.
Other physicians who commented on the trial were also enthusiastic. Renowned European interventional cardiologist Horst Sievert, MD of the Cardio-Vascular Center, Sankt Katharinen (Frankfurt, Germany) said that PROTECT AF is a "landmark trial." He added that these data will encourage him to implant the Watchman in patients who cannot tolerate warfarin.
Press conference moderator Ralph Brindis, MD, clinical professor of medicine at the University of California San Francisco and the regional senior advisor for cardiovascular diseases for Northern California of Kaiser Permanente (Oakland, California) noted that "this is a potentially very important device for the treatment of atrial fibrillation."
