BB&T Contributing Editor
NEW YORK — Pink clouds of confetti exploded over Times Square, announcing the start of the 11th annual Revlon Run/Walk for Women's Cancers, just as coincidentally the doors opened to the late April/early May annual meeting of the American Society of Breast Surgeons (ASBS; Columbia, Maryland). Nowhere else has there been such an unintentional yet extravagant beginning to a surgical conference that addresses a cancer that is treated far differently than other cancers.
Unlike many cancers where the incidence is expected with increased age, breast cancer often strikes young, productive workers and mothers, dictating treatments that will allow them to return to, and survive in, their previously active life. The objective in treating breast cancer is to deliver the patient disease free with minimal cosmetic and sexual defect concerns that are typically not echoed by other types of cancer treatments. The ASBS annual meeting is the only medical meeting dedicated exclusively to the latest issues affecting surgeons who treat breast disease, from diagnosis and staging to treatment planning and ongoing management.
According to the American Cancer Society (Atlanta), an estimated 178,480 new cases of invasive breast cancer were expected to occur in U.S. women during 2007. In addition, another 62,030 new cases of in situ breast cancer are expected, of which roughly 85% will be ductal carcinoma in situ (DCIS), a form that is currently being treated similarly as that of invasive breast cancer.
In the American College of Surgeons' (Chicago) National Cancer Database Benchmark Report, data indicate that 70% of eligible women with breast cancer will have a lumpectomy, while the remainder receives a mastectomy.
Breast-conserving therapy is preferable for many women because it provides superior cosmetic results compared to mastectomy, with similar patient survival rates when accompanied by lymph node testing and radiation therapy (RT).
The one issue still plaguing minimally invasive lumpectomies, however, is that of having to return to the operating room to remove cancerous tissue that was left behind during the initial lumpectomy.
Reduction of recurrence rate
Local recurrence of cancer in a patient that has had breast-conserving surgery (BCS) continues to plague this surgical advancement, with reports of 25% to 45% of all lumpectomies requiring a re-excision. Although the survival rate of BCS when coupled with radiation therapy is equivalent to that of mastectomy, the fact that so many patients end up returning to the operating suite haunts this advancement in breast cancer treatment.
Presented here for the first time were studies on a new radiospectroscopy intra-operative probe that helps detect positive tumor margins in order to avoid repeat surgeries, developed by Dune Medical Dev-ices (Caesarea, Israel). Using radio frequency spectroscopy technology that detects differences in the electromagnetic fields, the hand-held MarginProbe assesses margin malignancy status during an initial lumpectomy and helps avoid repeat procedures by enabling doctors to intraoperatively identify positive margins. As the tip of the probe is applied to excised lumpectomy tissue, radio frequency signals are transmitted into he tissue and when the signals are reflected back, they are analyzed using a specialized algorithm to determine the presence of malignant tissue.
In a randomized prospective multi-center study, the probe was used for positive margin assessment during breast-conserving surgery (BCS) and repeat surgery rates were compared in patients undergoing lumpectomy with and without the device. The double-armed study incorporated 11 sites, 35 surgeons, and 300 patients undergoing BCS and randomized the patients into two groups, one using the device as an assessment tool, the other utilizing conventional assessment tools. Use of the probe resulted in a 56% reduction in repeat lumpectomies with no statistically significant difference in the volume of tissue removed.
"This data is significant in demonstrating the potential of radio frequency spectroscopy in characterization of cancer at the resection margins," said Tanir Allweis, MD, Hadassah Hebrew University Medical Center (Jerusalem, Israel), an investigator in the study and presenter at the meeting. "The reduction of patient complications and discomfort as well as costs associated with repeat breast surgeries is of significant benefit to everyone involved in the treatment of breast disease."
Genomic-guided decisions
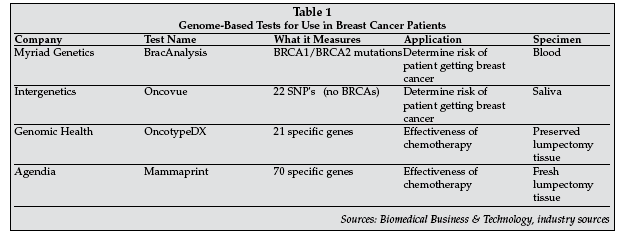
Although one in eight women will develop breast cancer, about 90% of the women who do develop breast cancer have no family history and of the 10% with family history, only 2% to 3% of those will develop it. So how does a women know if she is at risk or not? Several companies are now marketing risk assessment tools that use the patient's genes to determine both the chance of developing breast cancer as well as to predict therapy outcomes should she get it. Myriad Genetics (Salt Lake City) assesses a woman's risk of developing breast or ovarian cancer based on detection of mutations in the BRCA1 and BRCA2 genes. This test has become the standard of care in identification of individuals with hereditary breast and ovarian cancer and is reimbursed by insurance.
The ASBS Consensus Statement on BRCA testing states "Breast surgeons are in an ideal position to identify such high-risk individuals, to encourage and provide access for BRCA testing, and, most importantly, to help devise individualized management strategies for those who test positive."
Another company that incorporates both personal history and gene-based information to determine a woman's future breast cancer risk is Intergenetics (Oklahoma City). The company has developed a saliva swish test that is sent to its lab to determine non-BRCA genotype risks over the lifetime of the patient. With this tool, clinical medicine can focus attention on high-risk patients and employ more comprehensive screening tools, the use of preventive medicines and lifestyle changes that could lead to reduction of risk and the prevention of breast cancer.
Once a woman has been diagnosed with breast cancer, her treatment plan needs to be decided. Breast cancer patients can have markedly different treatment responses and overall outcome. While chemotherapy can reduce the risk of distant metastases by roughly one-third, 70% to 80% of patients receiving it would have survived without it. Gene expression profiles will outperform all currently used clinical parameters in predicting disease outcome following treatment.
According to Pat Whitworth, MD, associate clinical professor of surgery at Vanderbilt University (Nashville, Tennessee),"We have always treated 100% of the patients we felt required chemotherapy, knowing that only five will benefit from it. Now with genetic testing we can detect those five." The OncotypeDX test by Genomic Health (Redwood City, California) determines the likelihood of breast cancer recurrence in women with newly diagnosed, early stage invasive breast cancer as well as assesses whether the patient will benefit from chemotherapy.
This already is changing how physicians are treating breast disease and has the potential to significantly alter a patient's treatment plan. Traditionally, tumor characteristics such as size, lymph node involvement, and estrogen sensitivity have determined whether a patient receives chemotherapy. Studies now verify that cancer thumbprinting provides greater insight into the disease and test results should supersede the less-sophisticated measures used to assess the benefits of chemotherapy in the past.
In one study presented here, a comparison between recommended treatment plans with and without OnctoypeDX information was examined for 78 women with breast cancer. "Prior to this test, 39 out of 78 or 50% of the patients would have been recommended for chemotherapy, but using the OncotypeDX score only nine, or 12% of the patients were recommended for chemotherapy," said Leilia Thanasoulis, MD, of Bryn Mawr Hospital (Bryn Mawr, Pennsylvania), who added, "This analysis allows us to tailor breast cancer care to patients that can be spared or benefit from adjuvant chemotherapy."
The newest genetic entry, exhibiting here for the first time, was Agendia's (Amsterdam, the Netherlands) Mammaprint test that measures the activity of 70 genes using a fresh sample of tissue removed from a breast tumor and through computer analysis better directs the course of therapy. MammaPrint offers two results high risk and low risk and in studies accurately picked which women were at low risk for a recurrence at least 90% of the time. However, for women who were told they were at high risk for recurrence as a result of the test, just 23% experienced a relapse. This is the only FDA-cleared in vitro genetic test for breast cancer treatment (see Table 1 for a synopsis of in vitro genetic tests available for breast cancer).
New options for brachytherapy radiation therapy
Accelerated Partial Breast Irradiation (APBI) goes hand-in-glove with the trend towards minimally invasive lumpectomies. APBI allows only the tumor bed where it is estimated that 90% of the recurrences occur to be irradiated, as opposed to the full breast. APBI also takes only five days as opposed to the 30-day regimen required for full breast irradiation. For these reasons, patient demand for APBI has been high and has attracted several new competitors (see Table 2).
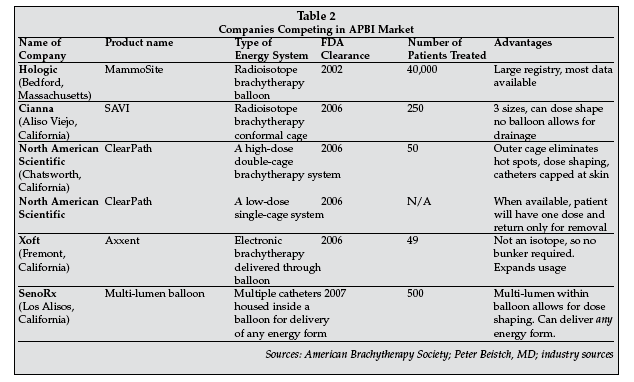
Peter Beitsch, MD, of the Dallas Breast Center, compared the various balloon catheters used for APBI in his presentation, "Partial Breast Irradiation: Balloon Catheters."
"It is always fun to see who exactly is in the audience," he began, and then polled the 350-plus attendees in the ballroom using the interactive polling system and found the following:
• 85% of the audience was surgeons.
• 80% to 100% of their practice was devoted to breast.
• 73% performed ultrasound-guided biopsies
• 38% performed stereotactic core breast biopsies.
• 32% were attending this meeting for the first time.
After a brief review of each brachytherapy balloon available, he said "Each device has an application that best suits certain patients," which suggests the concept of personalized medicine for the treatment of breast cancer an idea that seems to be developing in this space. His comments, along with company information on each brachytherapy balloon, are summarized in Table 2.
APBI technology is evolving rapidly, with new balloons and cages offering second-generation products with increased benefits for the patients. While current thinking is to provide high-dose radiation to the tumor bed over a brief period of time, there are at least two other schools of thought being investigated.
One is a single dose of radiation delivered during surgery, which Intraop Medical (Sunnyvale, California) and Karl Zeiss (Oberkochen, Germany) are developing, albeit with vastly different approaches.
Xoft (Fremont, California) also has the capacity to deliver intraop radiation and, according to Darius Francescatti, MD, assistant professor of surgery at Rush University Medical Center (Chicago) and surgical consultant for Xoft, "Xoft is more than a balloon it is a platform that has the capacity to do more than deliver brachytherapy. Xoft is in a position to deliver intraoperative RT without the requirement of bunkers, making it potentially available to many more patients."
The other novel approach is that of low-dose radiation delivered through the North American Scientific (Carlsbad, California) ClearPath cage that allows the patient to have an initial loading dose of radiation and return only at the end of the week to have the balloon removed, wearing a special barrier bra during this time. While waiting for these new technologies, RT treatments continue to be made more patient-friendly and available to a larger number of women with breast cancer.
SenoRx (Los Alisos, California), though the last to receive FDA clearance for its multi-lumen brachytherapy balloon, was blessed by the FDA with favorable labeling. Since some patients who could be candidates for balloon therapy are excluded because of the location of the lesion and their breast size, devices that can shift the radiation dose away from skin surfaces or ribs (dose shaping) can now be used in those cases.
The second-generation devices such as Contura, SAVI and ClearPath that have advanced multi-lumen designs may address this issue for certain patients. SenoRx was able to actually include this feature in their FDA labeling that states "Use of the appropriate off-set lumen(s) should be used to minimize the exposure to the skin," which highlights one of their key selling points.
Prophylactic mastectomies on the rise
With the advancement of risk assessment using the Gail model, family history, and now genotype studies, many women who are considered at high risk for developing breast cancer are now choosing to have a bilateral prophylactic mastectomy (BPM). In a breakfast workshop, Sarah McLaughlin, MD, of the Mayo Clinic (Jacksonville, Florida), discussed the "who, when and how" of this relatively new elective surgical concept.
Elective BPM often is at least considered by those women who carry the BRCA gene mutation and are at high risk. BRCA carriers have a 50% to 88% risk of getting breast cancer by age 70, significantly higher than a woman without BRCA. "In a study that followed women with the BRCA1 mutation, they found that those who had a BPM significantly extended their time to develop a cancer, and for breast cancer, the risk reduction was significantly reduced although not to zero, ranging from 94% to 100% risk reduction," McLaughlin said.
For those patients electing to have a contra-lateral prophylactic mastectomy at the same time as a therapeutic mastectomy is performed on the other breast, the data is unclear regarding long term survival. But the number of patients electing to do so is rising and "show no tendency toward plateauing anytime soon," McLaughlin said.
In a patient satisfaction survey conducted by the Mayo Clinic of 572 patients who underwent bilateral prophylactic mastectomy, after 14 years 70% were satisfied with their decision and 74% had a diminished level of breast cancer concern. "We do not want our patients to regret their irreversible decision," she said. "We asked the 19% who were dissatisfied with their choice what they wished they knew before the prophylactic mastectomy, and now try to incorporate that information into our counseling."
From better diagnostics to a wider range of therapies, breast cancer treatments are becoming a personal decision.
