BB&T Contributing Editor
WAIKOLOA Hawaii — The annual Hawaiian Eye Meeting, held recently at the stunning Hilton Waikoloa Resort, experienced record attendance, as specialists from today’s three critical areas of ophthalmology — cataract, refractive and retinal surgery — came from all over the U.S., attracted by the opportunity for education and just as obviously the chance to relax in the sunny and warm weather.
As the meeting got underway, course participants were greeted with the news that Bausch & Lomb (B&L; Rochester, New York), recently purchased by the private equity firm Warburg Pincus (New York) for $4.5 billion, was acquiring privately-owned eyeonics (Aliso Viejo, California). Although the purchase price was not disclosed, the conference was abuzz with speculation about a premium upfront cash payment and a potentially large earn-out based on future sales performance.
eyeonics has been enjoying tremendous growth recently with revenue for the year ended Dec. 31, 2007, of $34 million, virtually doubling the prior year’s revenue.
The success of eyeonics’ is partly attributable to the deft leadership of its co-founder and CEO Andy Corley, with virtually all of its revenue generated in the U.S. With purchase by B&L, this will likely lead to accelerated adoption of the crystalens IOL, given the ophthalmology giant’s global sales and marketing reach and its venerable brand name.
With the B&L acquisition, Corley will become its domestic surgical business president.
After the deal was unveiled, Corley noted that “through the extensive Bausch & Lomb sales and marketing organization, we expect to quickly and significantly expand the appreciation for the distinct patient benefits offered by the crystalens. We believe Bausch & Lomb’s deepened commitment to ophthalmology will further drive the crystalens IOL’s market acceptance as well as growth of the entire surgical product portfolio.”
Competing against two of the industry’s giants Alcon (Fort Worth, Texas) and Advanced Medical Optics (AMO; Santa Ana, California), as seen in Table 1, eyeonics has more than doubled its share in the premium refractive intraocular lens (IOL) market in the past two years, reaching an estimated 30% by year end 2007.
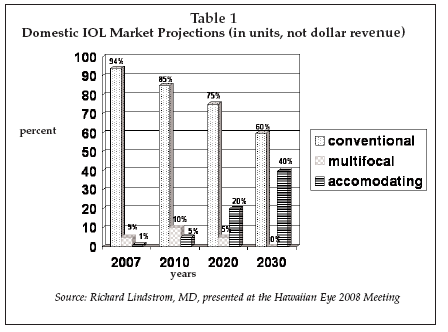
Growth path for IOLs rapid
This IOL market sector, which includes Alcon’s ReSTOR and AMO’s ReZoom multi-focal IOL brands, has been growing rapidly in the past few years. And Eyeonics has benefited from the November 2006 launch of its second-generation accommodating IOL, trade-named the crystalens Five-0, along with a sharp increase in its own sales force and the disenchantment among some surgeons and their patients with the clinical performance of ReSTOR and ReZoom.
The Five-O features a slightly larger diameter optic than its first-generation lens with rectangular plate haptics that allow for greater movement of the lens. It is also easier to implant and more stable within the capsule.
At the American Academy of Ophthalmology (AAO; San Francisco) meeting in New Orleans last November, eyeonics introduced its third-generation IOL, called HD-100. eyeonics is currently awaiting final FDA approval, based upon a PMA supplement filing last year.
The HD features a proprieta ry optics design that incorporates a small aspheric surface in the center of the optic. This appears to increase the depth of focus and, more importantly, improve near field reading vision.
The design of the HD has thus corrected the only major deficiency of eyeonics’ earlier products, which provided excellent intermediate (computer distance) and far vision but were mediocre in the near field.
In a talk titled “Update on the crystalens Five-0 Accommodating IOL,” Richard Lindstrom, a renowned cataract and refractive surgeon from Minnesota Eye Consultants (Minneapolis) and an adviser to several ophthalmic companies, noted outstanding clinical results in a recent small HD clinical trial. He reported that about 80% of the HD patients were enjoying superb reading abilities, while retaining excellent intermediate and far vision as well.
Lindstrom also said that HD patients are enjoying vastly improved contrast sensitivity (CS). (CS measures the ability to see details at low contrast levels and measures two variables: size and contrast.)
On the other hand, visual acuity, which is typically how devices are evaluated, measures only size.
In recent years, the ophthalmic community and the FDA have recognized the importance of CS as a true proxy for the efficacy of an IOL, and all IOL clinical trials incorporate contrast sensitivity as part of the trial requirements.
Touted: superior results
At an eyeonics-sponsored breakfast at the AAO meeting in November, Jay Pepose, MD, of Pepose Vision Institute (St. Louis), discussed this topic in detail, saying that both monocular and binocular testing revealed higher CS in eyes with Crystalens than either the ReSTOR or ReZoom lenses.
Concluding his talk, Lindstrom said, “I have no doubt that the HD provides superior results for my patients.”
Taking a somewhat controversial position, especially considering his role as a consultant to both Alcon and AMO, Pepose asserted, “I am going to switch all my future patients to the accommodating [i.e., eyeonics] IOLs and will not be using the multi-focal lenses anymore.”
Lindstrom’s comment are interesting to note relative to the sentiment of his colleagues.
A physician survey conducted at last year’s American Society of Cataract and Refractive Surgeons (ASCRS; Fairfax, Virginia) asked the question “Which (presbyopic IOL) do you want in your eye?” The most popular choice was crystalens at 43%, followed by the Synchrony lens (discussed below) at 33%, followed by ReSTOR at 16%, ReZoom at 8%.
At last year’s AAO, physicians were surveyed and asked two questions: “What is your most frequent choice today for presbyopia correction of cataract patients?” and “What do you expect will be your most frequent choice in ten years for presbyopia correction of cataract patients?”
The No. 1 choice today is multifocal IOLs but these lenses are expected to drop to number three in ten years, with accommodating IOLs expected to be the market leader
Another accommodating IOL is the Tetraflex, manufactured by Lenstec (St. Petersburg, Florida). According to Tetraflex’s principal investigator Paul Dougherty, MD, of the Jules Stein Eye Institute (Los Angeles), who presented here, this lens provides excellent vision and “has become my lens of choice for all my cataract patients.”
Tetraflex is a foldable acrylic lens that is easy to implant and is providing patients with excellent distance vision and enhanced near vision, compared to a standard IOL.
Lenstec recently completed enrollment in its domestic pivotal trial and will file a PMA application in the first half of 2009 after the FDA’s required one-year follow-up. This lens has the CE mark and is currently being sold in Europe and in several other countries.
Another accommodating lens, not specifically discussed here, is the Synchrony IOL developed by privately-owned, venture capital-backed Visiogen (Irvine, California).
The Visiogen device features a unique dual optic lens system with an easy-to-use pre-loaded injector. Synchrony has obtained a CE mark and in 4Q06 completed enrollment of its pivotal trial in the U.S. Based on that timeline, the company expects that it could be filing a PMA application in late 2008 or early 2009.
Excellent clinical data has been presented on Synchrony at prior ophthalmic surgical meetings, and it appears that this lens will become a significant competitor in the premium IOL space.
In another presentation later in the week, Lindstrom spoke on the topic of “Multifocal and Accommodating IOLs: Summary and Thoughts,” saying that he expected accommodating IOLs to “become the preferred option in the future.” As shown in Table 2, he predicted that accommodating IOLs — which accounted for a miniscule 1% of all IOL implants in 2007 in the U.S. — will rapidly garner market share in the coming years.
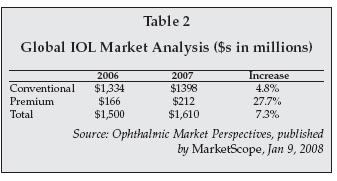
The key to Lindstrom’s projection is improved technology, which will lead to better “accommodative amplitude.” The premium IOL segment, which broadly-defined includes multi-focal and accommodation IOLs, as well as toric, aspheric and phakic lenses is boosting the growth of the global IOL market.
The Jan. 9, 2008, issue of Ophthalmic Market Perspectives, published by MarketScope (Manchester, Missouri), noted that worldwide premium IOL market unit sales have more than doubled in the past two years, and that, as noted in Table 3, 2007 worldwide premium IOL dollar revenue jumped nearly 28%.
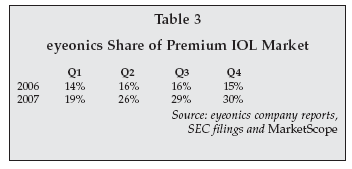
Conversely, the conventional or monofocal global IOL market grew less than 5% last year.
Opportunities in the retinal arena
Although the cataract and refractive sessions at the meeting were much larger than the retinal sessions, there was a lively and separate meeting for physicians focused on this specialty.
After being a moribund sub-specialty for many years, since the year 2000, there has been renewed vigor in the retinal world. The reason is simple — the introduction of three new medications for the treatment of age-related macular degeneration (AMD).
AMD is a degenerative eye condition associated with aging, and afflicting about 18% of those between 70 and 74 years old, then surging to 47% among those 85 and older. It is a chronic, progressive disease of the macula, the central part of the retina, causing irreversible loss of central vision and the No. 1 cause of severe vision loss and blindness among those over age 50 in the developed world.
With each successive introduction, beginning with Visudyne in 2000, followed by Macugen in late-2004 to Lucentis in mid-2006, the success rate for treating late stage (wet) AMD has dramatically improved the outcomes for elderly patients suffering from this dread disease.
Lucentis, a potent anti-VEGF agent introduced by Genentech (South San Francisco), has had an enormous impact on the treatment of AMD. Its clinical trials have demonstrated that Lucentis can not only halt the progression of AMD but that in a significant number of patients it has been shown to actually reverse its progression and substantially improve visual acuity.
Price (no surprise) the decider
Despite its stellar results, Lucentis has its shortcomings. Specifically at $2,000 per dose, it is extremely expensive. The regimen for many patients may mean treatments for several years.
The enormous cost of Lucentis has spurred the off-label use of another Genentech FDA-approved compound Avastin, which is a very similar molecule to Lucentis and widely used as an anti-cancer drug. The average cost for Avastin to treat AMD is about $50 per dose, a paltry 2.5% of the cost of Lucentis.
In October 2006 the National Eye Institute (NEI) of the National Institutes of Health, in response to a huge controversy surrounding the relative safety and efficacy of Lucentis and Avastin and the huge disparity in their cost, announced that they would be launching a clinical trial comparing the outcomes of Lucentis and Avastin. Patient enrollment for the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) began before the end of 2007.
The NEI study plans to enroll 1,200 patients with newly-diagnosed wet AMD at 47 centers in the U.S. and will follow patients for two years. It is expected to take about four years to complete, with the first data, at one year follow-up, expected to be released in late-’09.
During one of the sessions here in Hawaii, retinal physicians were polled on their use of Lucentis vs. Avastin for wet AMD. Not surprisingly, the doctors said that they used Avastin 69% of the time and Lucentis only 25%. The other 6% was accounted for by either Visudyne or Macugen.
This outcome is consistent with the feeling in the retinal community that the drugs are essentially the same and that the deciding factor for selection is price. Clearly, the CATT data will be very welcome information.
Despite the dramatic improvement in AMD drugs, the search for better treatment modalities continues. One approach, which was first introduced at last year’s Hawaiian Eye Meeting was presented again, with updated clinical data.
An AMD device strategy
Michael Ip, MD, associate professor of ophthalmology at the University of Wisconsin (Madison) and an active clinical investigator, discussed a novel device-based approach to AMD. Developed by NeoVista Inc. (Fremont, California), the Epi-Rad90 Ophthalmic System features the use of an ionizing radiation source that delivers strontium-90 directly to the macula, or central zone in the retina.
At the outset of his talk, Ip noted the very impressive results of Lucentis and asked, “Why do we need to revisit radiation as a potential treatment for age-related macular degeneration?”
His answer, that radiation therapy has strong anti-angiogenic, anti-inflammatory and anti-fibrotic effects and can work synergistically with oncolytic compounds.
For example, it is very beneficial in combination with Avastin for colorectal cancers. In addition, he indicated that the safety of strontium-90, particularly at relatively low doses, has been well-demonstrated.
He also pointed out that past results of using radiation therapy to treat AMD has been mixed. “Some studies have been positive, while some studies have been negative.”
The goal of this therapeutic approach is to permanently disable the proliferating new cells — the ultimate culprits in causing central vision loss — by damaging their DNA structure.
The procedure delivers strontium-90 at a controlled penetration depth of about 3 mm, irradiating the area where new vessels — known as neo-vascularization — is occurring. It is minimally invasive and is performed under local anesthesia during a partial vitrectomy, a common surgical procedure for retinal specialists, and takes about 30-45 minutes in total treatment time.
The latest Epi-Rad clinical results, with 24 month follow-up data, were presented here by Ip. In the study of 18 patients, 89% of patients lost fewer than 15 letters, 50% gained more than zero letters and 17% gained 15 or more letters.
He also showed there was no evidence of radiation toxicity at two years.
He called this data “compelling” in both safety and efficacy measures and noted that it compared very favorably to the stellar results seen in two key Lucentis pivotal trials — ANCHOR and MARINA.
After reviewing one-year results of a study previously reported at the November 2007 AAO meeting that used this radiation therapy concomitantly with Avastin, Ip said that “ by permanently disabling the proliferating CNV cells, we may be able to reduce the number of anti-VEGF treatments that we do.”
Indeed, one of the key findings from this study was that 85% of the patients did not require additional injections after their initial injections.
As shown in Table 4, presented by Ip, there appears to be several potential advantages of the NeoVista device. Perhaps the most critical attribute of this therapy is that it appears to boost the durability of VEGF compounds, thus potentially reducing the need for these costly and inconvenient monthly injections.

Following Dr. Ip’s talk, the moderator for this session, noted retinal surgeon Elias Reichel, MD, from Tufts New England Medical Center (Boston), said that “there clearly does seem to be an opportunity here where we can reduce the number of treatments.”
Needed: cost reduction
A poll conducted at this meeting, aimed at assessing the need for ongoing Lucentis injections showed that the average patient requires about 4.5 injections per year. While it is far too early to tell how much Epi-Rad will reduce the frequency of injections, it does appear likely that it will have a positive impact in this area.
Each monthly Lucentis injection costs the healthcare system about $2,200 (drug cost plus the physician injection fee). Including a monthly patient out-of-pocket, co-pay cost of $440, the annual cost is about $26,000 per patient, with the out-of-pocket annual cost of about $5,000.
A less obvious but important benefit of reduced injections is that it eliminates the risk of endophthalmitis. This dreaded complication of all intraocular surgeries, although extremely rare, can result in the possible loss of vision.
In another talk, Ip reported that endophthalmitis rates in key AMD trials such VISION, ANCHOR and MARINA were 0.1%, less than 0.1% and 0.05% respectively.
NeoVista is in the midst of its pivotal clinical trial, dubbed the CNV Secondary to AMD Treated with Beta Radiation Epiretinal Therapy (CABERNET) trial and hopes to complete it by 3Q08. The trial is being conducted at roughly 30 sites worldwide (20 in the U.S.) and is randomizing patients to either strontium-90 plus Lucentis or to just Lucentis. A total of 450 patients will enter the trial, 300 on the NeoVista arm and 150 in the Lucentis arm.
If that schedule is met, NeoVista hopes to submit its PMA in the third quarter 2009 and begin U.S. commercialization about one year later. It also hopes to win the CE mark sometime this quarter.
NeoVista, founded in 2002 by The Innovation Factory (Duluth, Georgia), has been funded by a prestigious group of venture capital firms since its inception. It is now well-funded to complete its pivotal trial and begin commercialization.
