Cardiovascular Devices & Drugs Contributing Editor
BOSTON — How often do you hear one of the world’s most renowned and veteran physicians describe the state of knowledge in his own subspecialty as “overwhelmingly ignorant” — especially if that physician is the organizer of an outstanding annual conference focused on that subspecialty?
Yet, that is the comment made to Cardiovascular Devices & Drugs by Jeremy Ruskin, MD, director of the Cardiac Arrhythmia Service at Massachusetts General Hospital (Boston), a world-famous electrophysiogist, and founder of the highly regarded Boston Atrial Fibrillation Symposium (BAFS), held here in mid-January.
Elaborating on this extraordinarily candid comment, Ruskin said, “I think that the progress in the field of AF has been truly stunning, but we still have a long way to go to fully understand this very complex arrhythmia and to treat it more effectively.”
Begun 13 years ago — with just 100 attendees in that inaugural year — BAFS has grown dramatically. Nearly 1,300 physicians, electrophysiology (EP) industry clinicians, entrepreneurs (both actual and aspiring) and the investment community attended this year, sitting through live cases, all-day lectures from the world’s most knowledgeable physicians treating atrial fibrillation (AF) and a potpourri of educational programs running late into the evening.
Why is BAFS attendance growing so? The simple answer: AF, the most common sustained rhythm disturbance and the most perplexing cardiac rhythm facing physicians.
Described by several physicians here as a “progressive chronic atrial cardiomyopathy,” AF, in terms of understanding its causes, mechanisms of actions and optimal treatment modalities, is still in an embryonic state. This discouraging fact, despite the millions spent annually by industry and academia, was obvious during BAFS, greatly frustrating symposium attendees — though offering potentially rich opportunities for device and drug developers, as suggested by the AF market, worldwide (see graphic, below).
It had long been estimated that about 2 million Americans were afflicted with AF. However, an article titled “Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence,” in the July 11, 2006, issue of Circulation, argued that its prevalence has been significantly underestimated.
The authors put its actual prevalence at more than 5 million, and with the growing numbers of aging Americans, this number is expected to skyrocket to nearly 16 million by 2050.
The Atrial Fibrillation Foundation (Reading, Massachusetts) has said that while AF afflicts a modest 2.3% of the population over the age of 40, it occurs in about 6% of those over 65 and nearly 10% of those over 80.
Remarkably, no approvals
Remarkably, despite the lack of consensus concerning optimal AF treatment, a large number of procedures are performed to treat the condition. CD&D estimates about 40,000 percutaneous catheter ablations performed in the U.S. in 2007 — a 40% to 50% increase over 2006 — and being done despite the fact that not one of the companies developing cardiac ablation systems have won no FDA approval for this specific application.
Catheter procedures are very lengthy, are poorly reimbursed, have mediocre clinical outcomes and may have disastrous results. But because AF is a chronic disease, many patients are seeking a permanent “fix,” not the palliation and onerous adverse effects produced by drugs.
About 25,000 AF surgical ablations concomitant to other open heart surgeries were performed in the U.S. in 2007. And a “stand-alone” AF ablations market is now emerging, with about 3,000 performed in 2007.
Basically, with a minor exception, companies selling surgical AF ablation devices are marketing them without the benefit of FDA approval for cardiac tissue.
Thus, about 65,000 “off-label” procedures were performed in the U.S. last year, reflecting, in part, the woeful failures of medical (that is, drug) management. At a symposium titled “Surgical Management of Atrial Fibrillation,” moderator Ralph Damiano, MD, chief of cardiac surgery at Barnes-Jewish Hospital (St. Louis), and one of the world’s most highly-regarded cardiac surgeons, said that “perhaps, in 10 years, we will totally understand [AF].”
What’s ‘best’ — not known
The statement, though specifically referring to the current state of cardiac surgery, echoed the sentiments of many physicians at the conference concerning AF in general.
Lending further murkiness to the discussions on AF in general and surgical ablation in particular, was a talk titled “Is There a ‘Best’ Energy Source for Surgical Ablation?” from David Kress, MD, a cardiac surgeon from the Midwest Heart Surgery Institute (Milwaukee).

“All energy systems have their advantages and shortcomings,” he said, and “the best” energy source will not be known until all have been subjected to the rigors of a well-designed clinical trial. Those results, based upon the clinical trials currently underway, appear to be several years away.
In yet another example of the lack of clarity for AF therapy, Marc Gillinov, MD, a noted cardiac surgeon at the Cleveland Clinic, spoke on the topic “The Left Atrial Appendage,” asking the question: “Should the left atrial appendage [LAA] be removed or occluded?”
His answer, not surprisingly, was “maybe.”
Gillinov’s pros and cons ranged from “it is there for a reason,” to “we can certainly live without it.” The LAA is generally believed to be the outflow location of micro-emboli that can develop in the left atrium.
This occurs very much more commonly in AF patients, whose atria are not pumping efficiently, thus allowing blood to pool and clots to form. An LAA occlusion device can be viewed as a non-pharmacological stroke-prevention strategy for AF patients. Discussing two new LAA devices, Gillinov termed them “promising.”
Both the Cardiac Surgery division of Medtronic (Minneapolis) and Atricure (West Chester, Ohio) are conducting human clinical trials for occlusion devices that appear to be simple to use, rapidly deployable and minimally invasive.
Atricure, with 11 human cases completed to date in Switzerland, submitted its 510(k) in March 2007, and analysts are predicting FDA approval in the next several months.
Closing out this session, Damiano asked: “Where Will Standalone AF Surgery Be in Five Years?” And he specifically asked the panelists: “Will you be doing more AF surgery or less?”
Ebullient vs. restrained
Cardiac surgeon James Edgerton, MD, from the Texas Hospital of the Southwest (Plano), developer of the largest and most impressive AF surgical ablation data, was ebullient about the future of AF ablation. “We will be doing a ton of patients,” he said. The other panelists were far more restrained, however, saying that, in the future, surgeons are likely to play a much more modest role in AF treatment.
Damiano’s challenged that, saying: “If we get far better data than we have now, the role of AF surgical ablation is bright.”
The topic of how electrophysiologists and cardiac surgeons will work together to treat AF patients is an important one as therapy options improve. These two subspecialties are not natural allies and often vie for AF patients.
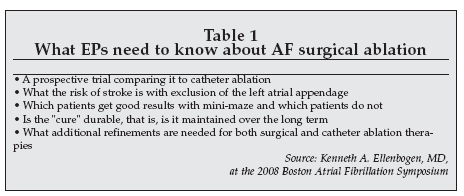
A lunch meeting titled “Electrophysiologic Perspective on the Bipolar Epicardial Ablation Procedure for Atrial Fibrillation,” supported by an educational grant from Atricure, featured Kenneth Ellenbogen, MD and director of clinical cardiac, electrophysiology and pacing at Virginia Commonwealth University (Richmond, Virginia) as one of the speakers.
Ellenbogen, who was the attending EP during a live AF surgical ablation procedure shown at BAFS, spoke on the topic of “Patient Selection for Epicardial Ablation,” and stated that “having the ability to offer a broad range of therapeutic options is the right course in treating atrial fibrillation.”
He was very supportive of the surgical approaches to AF intervention, saying that the results have been “surprisingly good.” However, he indicated that there is much that EPs need to know before fully supporting surgical ablation therapies.
These are requirements outlined in Table 1
Evidence-based ‘murk’
Further evidence of the murky world of AF treatment options was highlighted in a talk by Karl-Heinz Kuck, MD, from the Asklepios Klinik St. Georg (Hamburg, Germany).
The topic “Update on Clinical Outcomes of Balloon-Based Atrial Fibrillation Ablation,” discussed the shortcomings of widely-used radio frequency (RF) energy and provided an excellent overview of three key energy sources — cryo, laser and high intensity focused ultrasound (HIFU) — that used to perform ablations within the pulmonary vein (PV). Kuck, one of Europe’s most esteemed EP specialists, said he “strongly [believes] that RF energy to treat the pulmonary veins has limited benefits and we need a simpler anatomical approach.”
Many industry experts are enthusiastic about balloon-based AF ablation because it appears to be safe and requires no intra-procedural imaging. However, Kuck said that it can be technically challenging and has a long learning curve.
While his overall review of this area was positive, Kuck noted that these approaches are not yet perfected and that “further technical and procedural improvements are in the pipeline.”
Privately-owned, venture capital backed CardioFocus (Marlborough, Massachusetts) is the developer of a multi-lumen catheter that is shaped to reside in the pulmonary vein orifice. It delivers laser energy from a continuous wave 980 nanometer solid state diode laser. One advantage of this approach is that it enables direct visualization because an endoscope is used in conjunction with the procedure.
The company is currently enrolling patients in its U.S. pivotal clinical study with paroxysmal (intermittent) atrial fibrillation in the Endoscopic Ablation using Light Energy (ENABLE) trial. The company is in the early stages of the enrollment phase with 20 centers up and running.
Kuck said that he has been involved with this technology in a single-arm European trial, which has enrolled 30 patients. He indicated that about two-thirds of the patients were free of AF at one-year follow-up. His concluding comment on this modality is that it is “complex.”
Ablation by balloon
HIFU is being investigated by another private company, ProRhythm (Ronkonkoma, New York). Its technology involves the use of a dual balloon catheter design which creates a parabolic gas-fluid interface that reflects and vectors “focused” ultrasound to create a circumferential lesion around the PV. The energy delivery takes between 40 and 90 seconds and unlike RF energy, HIFU is non-thrombogenic.
Whereas RF catheter AF ablation procedures can take a few hours, procedures with the company’s third generation product, which features a wider focal zone, are considerably shorter. This is clearly a significant advantage for EP cath labs, physicians and their patients. The company has CE-mark approval and is in the midst of a controlled launch for the third generation product. In the U.S., it is in the early stages of its pivotal trial, which is being conducted at 25 centers and will eventually enroll 250 patients.
The recent approval by the FDA to allow HIFU to be offered as first line therapy in the trial is expected to accelerate patient enrollment, which had been sluggish under the formerly rigorous inclusion criteria.
The public company CryoCath Technologies is perhaps the best known balloon-based catheter ablation company, with its Arctic Front cryoablation balloon. This bi-directional, double balloon catheter uses cryothermia as its energy source and is specifically designed to treat paroxysmal atrial fibrillation.
The Arctic Front has received a CE mark, and as of the end of 2007, over 1,600 procedures had been performed in Europe. In the U.S., its pivotal Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) clinical trial has now enrolled about 200 patients, with a goal to completing the required full enrollment sometime in the second quarter 2008. A one-year follow-up will be required before filing a PMA with the FDA.
Arctic Front clinical data reported so far has been very positive. The second cohort of 18 patients in the feasibility trial, which were treated with its most current catheter technology, were free of AFib at six months. Three of the patients required a second procedure, which is permissible under the protocol set for the trial.
Of these three patients, one is still receiving anti-arrhymthmic drug (AAD) treatment. All of the remaining 17 patients are off AAD’s. No serious adverse events were reported in this small trial.
Looking to the future
Given the tremendous need for new technologies to detect and improve AF, it is not surprising that many innovative therapies are under development.
In a session titled “Future Technologies for AF Management,” the top three abstracts were presented orally to the full audience. These three technologies were selected from a total of 34 submitted abstracts and 25 posters. All three were private companies, in various stages of development of their technology.
Voyage Medical (Campbell, California), a relatively new startup that is venture capital-backed, has developed a visualization system which it calls the IRIS Direct Visualization System. This technology has broad application as both a diagnostic and therapeutic platform, although its initial application will be for trans-septal punctures that are a pre-requisite before left-sided AF catheter ablation.
Trans-septal punctures are a challenging aspect of any left-sided procedure, with a complication rate that the company estimates at about 1%. These complications can be serious and potentially life threatening. For example, tamponade, a condition where fluid collects between the heart muscle and the pericardial sac that envelopes the heart, can cause death if not treated quickly.
In addition to dramatically lowering the risk of a serious adverse event, the company expects that it will significantly reduce the time that a trans-septal puncture currently takes, especially for less experienced operators. Its first-in-man trans-septal procedures were recently performed outside the U.S. The company has submitted its 510(k) application and is hoping for an FDA approval in the near future.
The Voyage platform will serve in AF ablations by being able to directly visualize in high resolution the efficacy of lesion delivery, preventing while assessing and optimizing therapy at critical sites, including contiguity. It also is expected that the company’s technology can be used in interventional cardiology procedures such as septal defect identification and closure, transcatheter valve replacement and repair and the placement of leads associated with ICD devices.
The robotic approach
Another private company developing novel AF technology is CyberHeart (Menlo Park, California), which recently obtained its first round of venture capital financing. This non-invasive robotic stereotactic radiosurgery system technology, called the CyberKnife, was originally brought to market several years ago by publicly-owned Accuray (Sunnyvale, California) for the non-invasive treatment of various body tumors. It has been successful in that realm, as shown by Accuray’s meteoric revenue growth and unit placement growth.
CEO Patrick Maguire, MD, described the company’s technology as “a radiosurgical ablation device that accurately tracks, targets and corrects for motion during treatment, with real time image guidance to deliver ablation using robotics.”He clearly differentiated it from radiation therapy which treats normal tissue and radiosurgery, which is focused ablation energy.
In April 2007, Accuray entered into an agreement with CyberHeart whereby the latter is modifying Accuray’s technology to develop a non-invasive method for performing various cardiac ablations.
In the event CyberHeart is able to successfully develop and commercialize such a system, Accuray will be the supplier of radiosurgery equipment to CyberHeart and will also be entitled to receive specified payments based on usage of the CyberHeart system.
Broader cardiac applications
This technology has the potential to be used not only for AF ablations but other cardiac diseases that could benefit from accurate lesion placement.
For example, an article in the December 27, 2007 issue of The New England Journal of Medicine” titled “Prophylactic Catheter Ablation for the Prevention of Defibrillator Therapy,” discussed the benefits of catheter ablation of arrhythmogenic ventricular tissue as a means to reduce the painful and often unnecessary shocks of ICD devices.
CyberHeart’s non-invasive approach to creating lesions could potentially be very useful in this situation. The company has successfully completed some AF animal trials and hopes to initiate its human clinical trials later this year or in early 2009. As shown in Table 2, there appear to be several potential advantages of the CyberHeart AF technology.

A third company that presented at this session was Tissue Sensing Technologies (New York), which is addressing the challenge of improving the efficacy and efficiency of RF lesion creation. Its technology measures the electrical coupling that occurs between the RF electrodes and the cardiac tissue, thus indicating how good the ablation contact is.
Presenter Larry Chinitz, MD, director of clinical cardiac electrophysiology at the Heart Rhythm Center at New York University Medical Center (New York) stated that “contact will be a big part of the future,” particularly because of the introduction of irrigated RF catheters. “We need better assurance of contact with the tissue and the force of contact is not predictive of lesion depth,’ he said. One of the advantages of this technology, which creates more detailed heart chamber geometry, is that it could be integrated into existing mapping and catheter navigating equipment such as the St. Jude Medical (St. Paul, Minnesota) EnSite system.
