BB&T Contributing Writer
LOS ANGELES — The 49th annual meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO; Fairfax, Virginia), held here during the last few days of October, enjoyed record attendance as its nearly 9,000 members are experiencing the benefits of an explosion in new technologies. Founded in 1958, ASTRO is the largest radiation oncology society in the world, its exhibit hall featuring a plethora of companies from both the pharmaceutical and device sides of this therapeutic world.
The meeting just follows the recent online release of heartening news from the National Cancer Institute of the National Institutes of Health. The death rate from cancer, which had been dropping by an average of 1.1% a year since 1993, has accelerated further recently, to an average decrease of 2.1% a year from 2002 to 2004, the latest dates for which these statistics are available.
Despite this decline, however, cancer remains — after heart disease — the second leading cause of death in the U.S., expected to total about 559,650 this year. But progress in improving cancer therapy is advancing on many fronts.
For breast cancer, less-invasive breast conserving surgery (lumpectomy) and post-op accelerated partial breast irradiation (APBI) have made a significant impact. PBI has been challenging whole breast, external beam radiation in the past several years for post-lumpectomy patients. Consistent with the growth of less invasive surgery in other surgical specialties, this trend is accelerating with the strong marketing and positive long-term data that has been released by industry pioneer Cytyc (Marlborough, Massachusetts). Cytyc, a leading player in the women's health field, was acquired for $6.2 billion by Hologic (Bedford, Massachusetts) in late October.
A shorter course
The main advantage of APBI, often referred to as breast brachytherapy, is that it features a much shorter course of therapy than whole breast radiation (typically five days vs. six to eight weeks), with similarly low rates of cancer recurrence. Data from the American Cancer Society (Atlanta) indicates that there will be 178,000 new breast cancer cases diagnosed in the U.S. in 2007. Industry sources estimate that about 50% of these cancers are early stage and therefore are potential candidates for breast-conserving surgery and follow-up APBI.
According to a May 2007 research report written by Jason Mills, medical device analyst at Canaccord Adams (Vancouver, Canada), the number of breast brachytherapy procedures in the U.S. will grow from an estimated 13,000 performed in 2006 to 47,000 performed in 2009, representing a torrid compound annual rate of 60%. Mills estimates the 2007 market for breast brachytherapy at $44 million, but puts the future market potential for this technology at around $350 million.
While the Hologic product, trade-named the MammoSite, currently dominates this category, several new competitors — with variations on the same theme of local, post-lumpectomy irradiation — are hoping to challenge its supremacy.
These contenders include SenoRx (Aliso Viejo, California), Xoft (Fremont, California), Cianna Medical (Aliso Viejo, California) and North American Scientific (NAS; Chatsworth, California). Each of these companies had prominent exhibits at ASTRO, and several held evening symposia.
'... 50 ways to irradiate'
NAS, which is a strong player in the prostate brachytherapy market with its palladium and iodine seed offerings, was one of the companies that held an informational evening meeting at ASTRO.
The company's new ClearPath HDR is similar in some respects to other APBI devices such as MammoSite. However, with a design that features inner and outer multiple catheters, the company believes that it can expand the range of patients who can receive APBI. Therapeutic radiation dose can be distributed differentially to achieve full coverage of the treatment area, while sparing normal tissue.
At a NAS evening symposium, Rakesh Patel, MD, a prominent radiation oncologist practicing at the University of Wisconsin Medical School (Madison), gave a comprehensive and informative talk that he wryly titled "50 Ways to Irradiate the Breast."
After reviewing the pros and cons of other APBI devices, Patel praised NAS's product design, noting its features for improved patient comfort. He said that he favors this product "because it gives me the ability to achieve optimal radiation dosimetry."
Peter Beitsch, MD, a surgical oncologist at the Dallas Surgical Group (Dallas, Texas), also spoke highly of the ClearPath, saying that the product would be a valued addition to the armamentaria of the breast surgeon and radiation oncologist, who "need to work together for the maximum benefit of the patient."
Beitsch, a renowned breast cancer surgeon, has also noted in past ClearPath comments that "the ability to control the dose distribution should reduce toxicity to the skin and chest wall, decreasing complications and improving cosmesis."
Supporting the positive comments of Beitsch and Patel, Adam Dickler, MD, a radiation oncologist from Rush University Medical Center (Chicago), presented a paper titled "A Dosimetric Comparison of MammoSite and ClearPath HDR Breast Brachytherapy Devices." His conclusion was that "ClearPath HDR delivered, in every case, less radiation to normal tissue than MammoSite. Any decrease in the risk of normal tissue toxicity is significant for patients."
NAS currently is selecting patients at five clinical sites, in order to fully validate the product's capabilities. A full market launch is expected sometime in 2008.
Dose-shaping, reducing 'hot spots'
SenoRx is another prominent company addressing this market. Its Contura product, recently FDA-approved, is being utilized in select centers prior to a full launch in 2008. This device features multiple offset lumens inside of a balloon. The applicator provides dose-shaping opportunities to minimize potential toxicities to adjacent skin, ribs and other critical structures while enjoying the important attributes of the balloon. The balloon allows the device to lift tissue away from the source to reduce radiation "hotspots" and also fixates the geometry of the tissue to maintain critical reproducibility of dose from treatment to treatment.
The Contura also offers vacuum ports to allow for the removal of air and accumulated seroma, which is a pocket of clear fluid that sometimes develops after breast surgery. The vacuum also aids in tissue to balloon conformance which is another critical aspect of appropriate dose delivery.
The company expects to undertake a full commercial market launch of Contura in early 2008.
SenoRx already markets to the breast cancer surgeon with its flagship percutaneous Encor vacuum-assisted breast biopsy system. This affords the company a natural competitive advantage, as both breast cancer surgeons and radiation oncologists play a key role in post-lumpectomy, APBI device selection.
Just prior to ASTRO, Cianna Medical (formerly Biolucent) reported that it had treated 100 patients with its product, trade named SAVI. This device is also a single-entry, multi-catheter applicator.
According to Constantine Mantz, MD, a radiation oncologist with 21st Century Oncology (Fort Myers, Florida) "SAVI is becoming a very important tool in the management of our breast cancer patients, because we can deliver breast brachytherapy to more patients ... " 21st Century Oncology operates 83 radiation therapy centers in 16 states.
All these new devices deliver radiation therapy locally as part of breast conservation therapy, an approach that is growing in popularity.
Accuray upgrades
This year's ASTRO was also highlighted by an impressive showing by Accuray (Sunnyvale, California), one of the industry's leaders. The company has developed and markets the CyberKnife robotic radiosurgery system, which integrates modern imaging technology, intelligent robotics, and high-dose radiation delivery, merged with complex algorithms to precisely target cancerous tumors.
This robotic system, typically selling for between $3.5 million to $4 million, employs a combination of real-time image guided technology with continuous target tracking and feedback, a compact x-band linear accelerator, a robotic manipulator arm, and a Synchrony Respiratory Tracking System.
Originally introduced to ablate brain tumors, the technology has been broadened and now can adeptly treat extracranial tumors, such as those occurring in the spine, lung, prostate, liver and pancreas.
In front of a standing room only crowd at their ASTRO exhibit booth and with a glitzy presentation that would have made any Hollywood producer proud, the company unveiled four technology advances that promise to significantly improve the clinical performance of its system.
During a radiosurgery procedure, clinicians can define the dose of radiation delivered during treatment using a collimator — a metal radiation blocking device with an opening that dictates the shape and size of the radiation beam. In the past, to vary the size and shape of their radiation beam it required the operator to manually switch out collimators in the middle of treatment. This slowed the treatment time considerably.
At ASTRO, Accuray introduced the Xchange robotic collimator changer, which eliminated the need to re-enter the treatment room, thus minimizing treatment delays.
This year's introduction of the Iris variable aperture collimator allows the CyberKnife system to efficiently deliver different sized beams from each treatment position. In addition to reducing treatment times, the Iris collimator enables clinicians to create and deliver treatments that have greater accuracy, more uniformity and better avoidance of nearby critical structures and healthy tissue than single collimator treatments. Another key upgrade is the 800 MU/min LINAC, capable of delivering higher dose rates of radiation to a tumor, thereby reducing treatment times.
Two other system improvements that debuted at ASTRO 2007 were the Sequential Optimization feature, which enables users to establish their own clinical objectives to achieve high quality, more intuitive treatment planning in a shorter amount of time and the RoboCouch patient positioning system with seated load. The latter feature offers easier loading and unloading capabilities and provides added comfort during treatment, minimizes patient motion and resulting in fewer treatment interruptions.
The upshot of these upgrades, which are provided free of charge to Accuray's Diamond service levels customers (about 90% opt for this program), is that its customers will experience a significant improvement in patient throughput while patients will reap the benefits of sharply lower treatment times.
At a poster at the company's booth, these reductions were quantified and illustrated. As an example, prostate cancer treatment time will drop for 30% to 50%, depending on the type of regimen followed, while lung cancer therapy will decrease by 33%.
Prostate cancer appears to be very well-suited to the CyberKnife. At a presentation at its booth, Don Fuller, MD and a radiation oncologist from the CyberKnife Center of San Diego (San Diego, California) called the CyberKnife an "amazing system" that he has been using for about 18 months to treat prostate cancer.
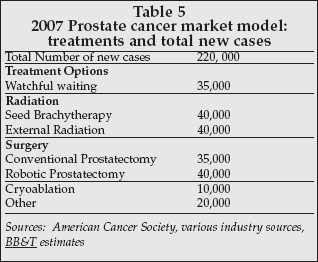
As shown in Table 5, relatively few prostate cancer patients have been treated by the CyberKnife and the bulk have been treated in the past two-to-three years. Fuller noted that despite this fact, 75% of his prostate cancer patients are selecting it over competing radiation alternatives such as seed brachytherapy or external beam radiation.
 |
'... blown away'
"Given the very limited clinical experience we have had to date, I am blown away by the high percentage of my patients choosing CyberKnife," Fuller said in his presentation. He speculated that patients were attracted to the significantly shorter treatment regimen that spans just five treatments over one week versus 40 treatments over eight weeks for external radiation techniques.
Testimony to the clinical successes and lucrative financial benefits generated by CyberKnife, Dr. Fuller and his eight radiation oncology colleagues will soon be opening up another CyberKnife center in nearby Encinitas.
Fuller will be the principal investigator of an Accuray-sponsored prostate cancer clinical trial that will be treating early stage organ confined cancers. It will compare the CyberKnife to HDR (high dose rate) temporary brachytherapy. It will treat a total of 330 patients, who will be followed for five years.
Another Accuray-sponsored prostate cancer trial will enroll an additional 330 patients and also do a five-year follow-up. The principal investigator will be Robert Meier, MD, a radiation oncologist from the Swedish Cancer Institute (Seattle), who has had considerable previous experience with the CyberKnife in treating prostate cancer. This trial will compare CyberKnife to a homogeneous dose distribution in early stage organ confined cancers.
Opportunity meaningful
Prostate cancer affords Accuray a meaningful opportunity. As Table 5 shows, t he prostate cancer therapy market is large, and the number of patients treated with radiation is significant. However, given the conservative nature of urologists and radiation oncologists, it probably will take the release of favorable five-year clinical data before CyberKnife for prostate cancer can really be widely adopted.
There is a lively debate with the radiation oncology community concerning the relative benefits of the CyberKnife compared to other companies who claim that their radiation therapy systems can produce comparable results at a reduced capital equipment cost.
About a week before ASTRO, Mark Jefferies, a research analyst with the investment banking firm Jefferies & Company (New York) hosted a conference call with two prominent radiation oncologists who are enthusiastic CyberKnife users.
Mark Brenner, MD and chief of the Department of Radiation Oncology at Sinai Hospital (Baltimore, Maryland) said that since acquiring the CyberKnife four and a half years ago, he has treated over 1000 patients."None of those patients would have been treated with other forms of external radiation systems," he said. Brenner went on to say that a comparison of the CyberKnife to other radiation-based systems is like "comparing apples to oranges."
John Kresl, MD, PhD, director of the CyberKnife Center at St. Joseph's Hospital (Phoenix, Arizona) ,echoed Brenner's ebullience, saying that he is very impressed by the "incredible level of accuracy and precision" he has seen using the CyberKnife.
