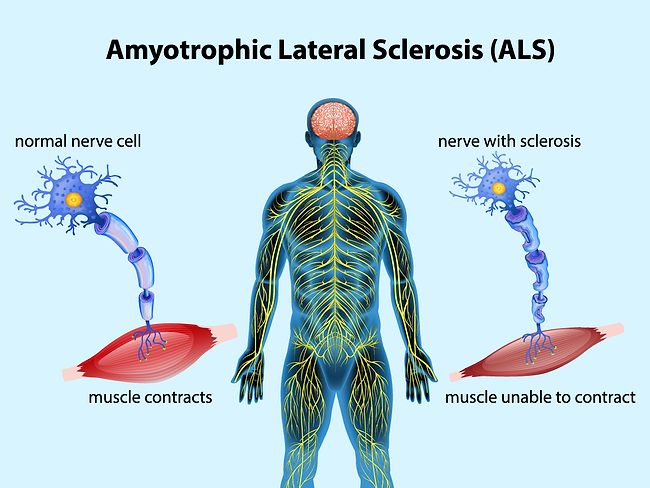
Articles Tagged with ''ALS''
Neurology/psychiatric
Sineugene’s TRIM72-targeted gene therapy for ALS gains IND clearance from FDA
Read MoreNeurology/psychiatric
Lifearc and Neuropeutics collaborate to advance small molecule for ALS
Read MoreNeurology/psychiatric