Science
Drug design, drug delivery & technologies
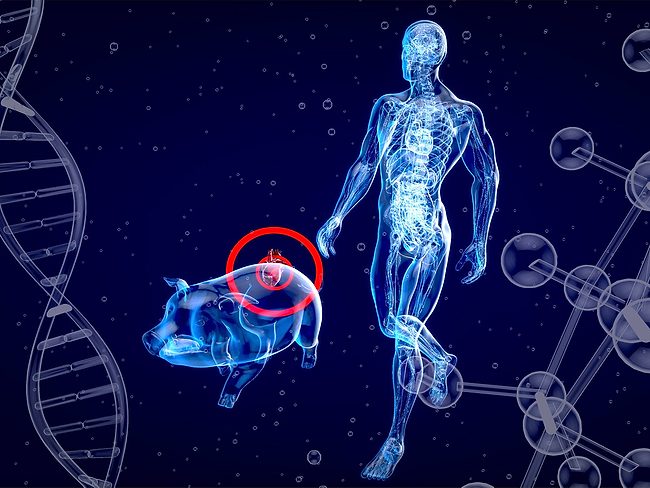
Ten days of normal survival of a pig liver in a human being
Read MoreDrug design, drug delivery & technologies
Ten days of normal survival of a pig liver in a human being
Read MoreDrug design, drug delivery & technologies