- BioWorld
- BioWorld MedTech
- BioWorld Asia
- BioWorld Science
- Data Snapshots
- Special reports
- Infographics: Dynamic digital data analysis
- Trump administration impacts
- Biopharma M&A scorecard
- BioWorld 2024 review
- BioWorld MedTech 2024 review
- BioWorld Science 2024 review
- Women's health
- China's GLP-1 landscape
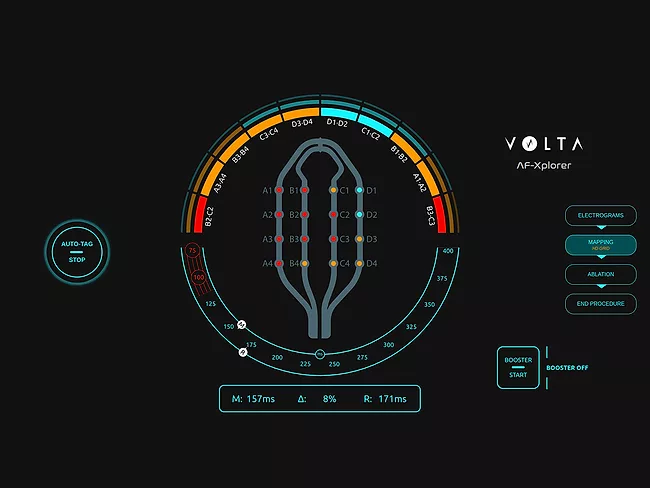
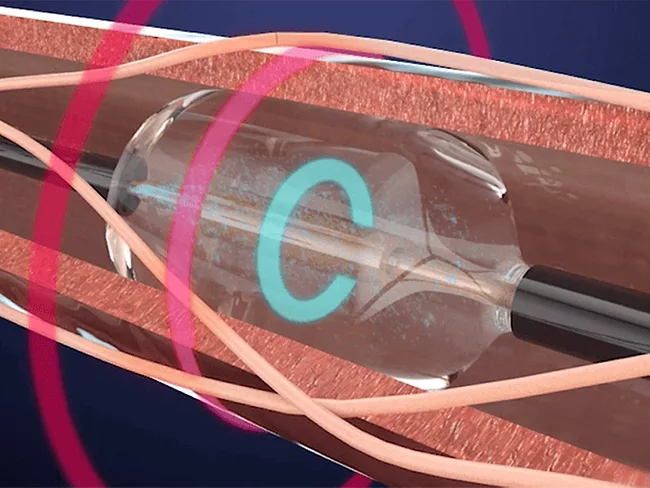
- PFA re-energizes afib market
- China CAR T
- Alzheimer's disease
- Coronavirus
- More reports can be found here
ARTICLES
EuroPCR 2024
Boston Scientific hoping to change EU guidelines for use of IVUS during PCI
May 23, 2024
EuroPCR 2024
Safety of Elixir’s Lithix Hertz contact lithotripsy catheter validated
May 16, 2024
EuroPCR 2024
Elixir’s Dynamx bioadaptor superior to Medtronic Resolute Onyx DES
May 14, 2024
- BioWorld
- BioWorld MedTech
- BioWorld Asia
- BioWorld Science
- Data Snapshots
- Special reports
- Infographics: Dynamic digital data analysis
- Trump administration impacts
- Biopharma M&A scorecard
- BioWorld 2024 review
- BioWorld MedTech 2024 review
- BioWorld Science 2024 review
- Women's health
- China's GLP-1 landscape
- PFA re-energizes afib market
- China CAR T
- Alzheimer's disease
- Coronavirus
- More reports can be found here