Med-tech startup Flow Forward LLC said it has raised a total of $7 million in its series A round. The news comes around the same time the private company reported completing two studies evaluating its Arteriovenous Fistula Eligibility (AFE) system for use in hemodialysis patients.
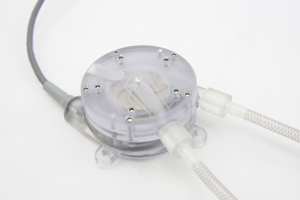
The Olathe, Kan.-based company's device is a small, minimally invasive blood pump system designed for temporary use to rapidly dilate peripheral veins through flow-mediated vascular dilation prior to arteriovenous fistula (AVF).
The AVF procedure establishes a direct connection between an artery and a vein during hemodialysis.
Nicholas Franano, founder, president and CEO of Flow Forward, said since the inception of hemodialysis procedures, there have always been issues with creating an access point where a dialysis machine connects with a patient's vascular system.
Franano previously served as CEO of Proteon Therapeutics Inc. The Waltham, Mass.-based company went public in 2014 and develops pharmaceutical vascular access solutions.
"There are still about a third of patients that can't get an AVF, mostly because they don't have a vein large enough in their arms to make an access site," Franano, told Medical Device Daily. "We want to enlarge veins in those patients and make them eligible for the surgery. But then if you look at the eligible population, those patients have a very high degree of initial failure, and they require additional surgery and angioplasty. Often the site is abandoned and another [entry point] has to be made."
Franano estimated the market for the device in the U.S. is $1.3 billion a year.
CAN'T STOP THE FLOW
In the last few months, Flow Forward has turned its efforts toward raising funding for the continued development of the pump. Flow Forward's series A funding will be used to complete additional modifications to the AFE system. Specifically the company is refining conduits that send a patient's blood in and out of the pump. The funding will also be used to upgrade the controller that drives the pump, a modification that Franano said will get the device closer to being ready for human testing.
Franano noted the firm has had tremendous investor support.
"The state of Kansas grants to our investors a tax credit worth 50 percent of their initial investment," he said. "That reduces a lot of the risk associated with investment."
Plans call for Flow Forward to raise between $8 million and $10 million in a series B round prior to the end of the year. Franano said the funding would allow the company to get through human clinical trials.
He added that no one else has this approach of dilating a vein prior to surgery and that three recently approved patents will provide long-term market exclusivity Flow Forward's products.
"The advantage is that we have the field to ourselves during the development process," Franano said. "The disadvantage is that we have to work hard enough to convince the nephrologists, vascular surgeons and vascular biologists that this is the right approach. I think we're well on our way to doing that."
MOVING TRIALS FORWARD
In a nonclinical study evaluating Flow Foward's device, ovine cephalic vein diameter increased on average 89 percent from 5.5 millimeters to 10.4 millimeters with treatment. After six weeks, both inflow arteries and outflow veins were larger in AFE system-assisted AVFs than in control AVFs, suggesting improved AVF maturation. Some preliminary results from this study were presented on Nov. 19, 2016 at the Veith Symposium in a presentation titled "Innovation in Vascular Access: Where Are We Headed?"
Flow Forward also reported the completion of a computation fluid dynamics (CFD) study in partnership with the research group of Yan-Ting Shiu in the Division of Nephrology & Hypertension at the University of Utah. This study used bench flow loops and CFD modeling to compare blood flow, pressure, wall shear stress (WSS), wall motion in AVF and AFE system outflow veins, and determined the effect of outflow vein diameter on these important biomechanical factors. Results show that peak WSS levels are very high in AVF outflow vein segments adjacent to the anastomosis, indicating a high likelihood of vein wall damage. This effect was especially pronounced with small veins. In the Flow Forward study, WSS levels were also elevated in AFE system outflow veins, but peak WSS levels were much lower than with AVF, indicating a lower risk of vein wall damage.
Flow Forward executives said they want to start a 10-patient clinical trial outside the U.S. next year. The company plans to have the technology on the European market by 2021.
"We'd like to be on the market in the U.S., in 2024," Franano said. "It's not near term, but it's progression."
